25 Aug, 2021 Sales for HIV drugs containing TAF to exceed $15bn by 2029 while TDF continues to decline, says GlobalData
Posted in PharmaHIV drugs containing tenofovir alafenamide (TAF) are set to see sales over $15bn by 2029*, supported by the ingredient’s high tolerance in high-risk patients, according to GlobalData. The leading data and analytics company compares this with drugs containing the once top-selling tenofovir disoproxil fumarate (TDF). These are set to see steady decline due to key disadvantages, including a link between these drugs and osteoporosis and kidney disease.
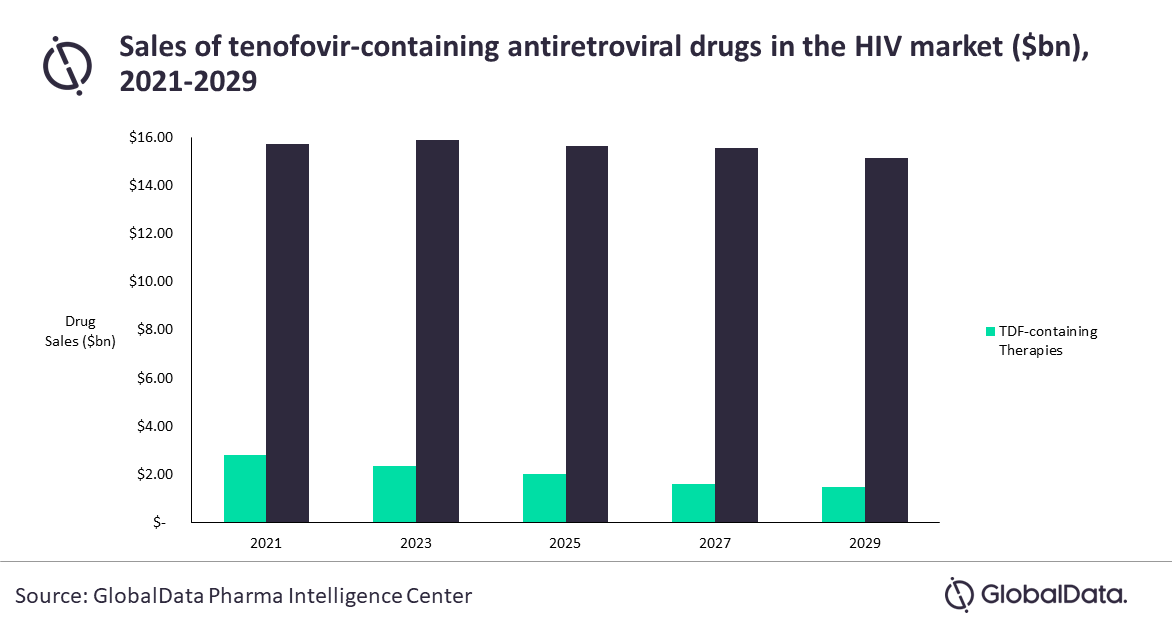
According to the latest report by GlobalData, ‘Human Immunodeficiency Virus (HIV) Therapeutics: Global Drug Forecast and Market Analysis to 2029’, TDF sales are expected to decrease from $3bn in 2021 to $2bn in 2029, while TAF remains consistently around the $15bn mark.
Magdalene Crabbe, MA, Senior Ophthalmology and Infectious Diseases Analyst at GlobalData, comments: “TAF is a lipophilic nucleoside reverse transcriptase inhibitor (NRTI) that is a constituent of some of the most commonly prescribed single-tablet regimens (STRs) for patients with HIV. TAF was preceded by TDF in the HIV market, and both products are hydrophobic structural analogs of naturally occurring nucleosides.
“For a number of years, TDF has been a component of the top-selling drugs in the HIV space. Gilead Sciences’ Atripla (efavirenz/emtricitabine/TDF) was the first once-daily treatment for HIV to launch in the US. However, the success of TDF has since been marred by links to osteoporosis and kidney disease. Renal toxicity has not been linked to TAF, and STRs containing this NRTI have been well tolerated by high-risk patients over the last six years.”
Despite the clear advantages of TAF-based products, drug-resistant strains of HIV remain a consistent threat to patients’ lives. Treatment with NRTIs is known to lead to multidrug resistance, especially in a therapy area where lack of compliance is common.
Crabbe concludes: “The reliance on treating patients with drugs that operate through one major mechanism of action is always a risk in situations where infected individuals must be treated for the rest of their lives.
“While it is clear that drug safety is a key consideration for pharmaceutical companies in this therapy area, enduring efficacy is of critical importance to ensure that patients have a good quality of life. It is increasingly possible that long-lasting success of TAF may fund drug developers’ efforts to manufacture innovative therapies that overcome multidrug resistant strains of HIV.”
*7MM: The US, France, Germany, Italy, Spain, UK and Japan
