Biopsy Devices – Pipeline Products by Stage of Development 19
Biopsy Devices – Pipeline Products by Segment 20
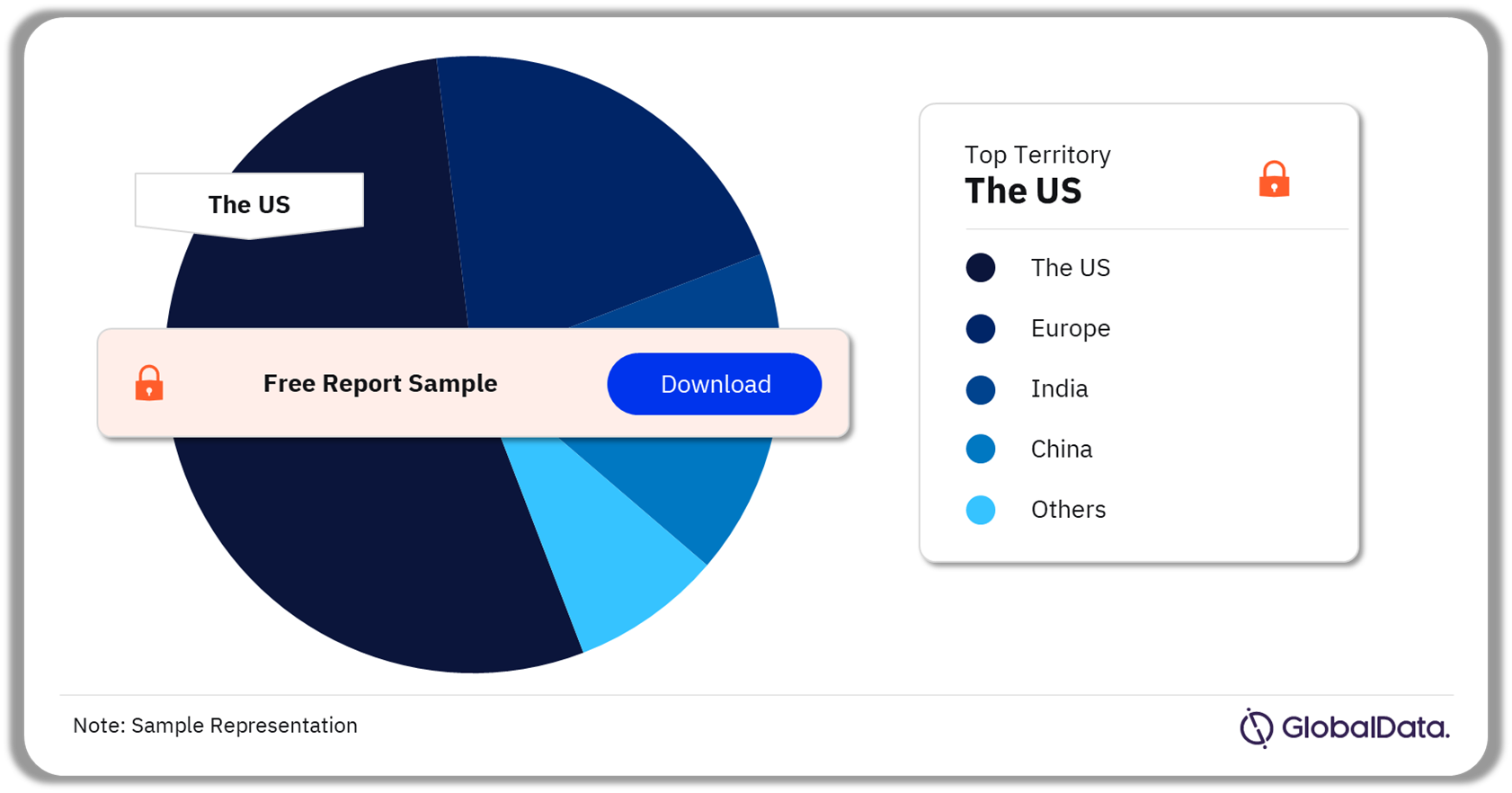
Biopsy Devices – Pipeline Products by Territory 21
Biopsy Devices – Pipeline Products by Regulatory Path 22
Biopsy Devices – Pipeline Products by Estimated Approval Date 23
Biopsy Devices – Ongoing Clinical Trials 24
Biopsy Devices Companies – Pipeline Products by Stage of Development 25
Biopsy Devices – Pipeline Products by Stage of Development 29
Actuated Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 33
OsteoAccess – Product Status 33
OsteoAccess – Product Description 33
Arch Medical Devices Ltd Pipeline Products & Ongoing Clinical Trials Overview 34
ARCH Pancreatic Lateral Sampling Device – Product Status 34
ARCH Pancreatic Lateral Sampling Device – Product Description 34
ARCH Prostate Transurethral Lateral Sampling Device – Product Status 35
ARCH Prostate Transurethral Lateral Sampling Device – Product Description 35
ARCH Pulmonary Lateral Sampling Device – Product Status 35
ARCH Pulmonary Lateral Sampling Device – Product Description 36
Becton Dickinson and Co Pipeline Products & Ongoing Clinical Trials Overview 37
Multi-Modality Vacuum Assisted Biopsy – Product Status 37
Multi-Modality Vacuum Assisted Biopsy – Product Description 37
BiBBInstruments AB Pipeline Products & Ongoing Clinical Trials Overview 38
EndoDrill GI (EDMX01) – Product Status 38
EndoDrill GI (EDMX01) – Product Description 38
EndoDrill URO – Product Status 39
EndoDrill URO – Product Description 39
BiBBInstruments AB – Ongoing Clinical Trials Overview 40
EndoDrill GI (EDMX01) – Pilot Study of EndoDrill Model X in Subjects with Suspected Muscle Invasive Bladder Cancer 41
Boston Scientific Corp Pipeline Products & Ongoing Clinical Trials Overview 42
EUS Guided Pulmonary BX – Product Status 42
EUS Guided Pulmonary BX – Product Description 42
Boston University Pipeline Products & Ongoing Clinical Trials Overview 43
Breast Biopsy Clip – Product Status 43
Breast Biopsy Clip – Product Description 43
Breast Biopsy Introducer – Product Status 44
Breast Biopsy Introducer – Product Description 44
Broncus Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 45
BioStar cTBNA/EBUS-TBNA Needle – Product Status 45
BioStar cTBNA/EBUS-TBNA Needle – Product Description 45
Carlos Health Institute III Pipeline Products & Ongoing Clinical Trials Overview 46
Automated Skin Biopsy Device – Product Status 46
Automated Skin Biopsy Device – Product Description 46
ClearPoint Neuro Inc Pipeline Products & Ongoing Clinical Trials Overview 47
MR Safe Smart Biopsy Needle – Product Status 47
MR Safe Smart Biopsy Needle – Product Description 47
MRI-Safe Myocardial Biopsy Forceps – Product Status 48
MRI-Safe Myocardial Biopsy Forceps – Product Description 48
CleveX, Inc. Pipeline Products & Ongoing Clinical Trials Overview 49
ClearVu Punch Biopsy – Product Status 49
ClearVu Punch Biopsy – Product Description 49
Columbia University Pipeline Products & Ongoing Clinical Trials Overview 50
Inner Ear Fluid Aspirator – Product Status 50
Inner Ear Fluid Aspirator – Product Description 50
OCT- Assisted Endomyocardial Biopsy Catheter – Product Status 51
OCT- Assisted Endomyocardial Biopsy Catheter – Product Description 51
Control Medical Technology LLC Pipeline Products & Ongoing Clinical Trials Overview 52
30ml ASPIRE 1 SPIN 170 – Product Status 52
30ml ASPIRE 1 SPIN 170 – Product Description 52
30ml ASPIRE 1 SPIN 90 – Product Status 53
30ml ASPIRE 1 SPIN 90 – Product Description 53
30ml ASPIRE 2 SPIN 170 – Product Status 53
30ml ASPIRE 2 SPIN 170 – Product Description 54
30ml ASPIRE 2 SPIN 90 – Product Status 54
30ml ASPIRE 2 SPIN 90 – Product Description 54
30ml ASPIRE Aspirator – Product Status 55
30ml ASPIRE Aspirator – Product Description 55
CR Bard Inc Pipeline Products & Ongoing Clinical Trials Overview 56
EnCor Mini – Product Status 56
EnCor Mini – Product Description 56
Minimally Invasive Cutting Instrument – Product Status 57
Minimally Invasive Cutting Instrument – Product Description 57
Crux Medical Innovations Pipeline Products & Ongoing Clinical Trials Overview 58
Multiple Core Biopsy Device – Product Status 58
Multiple Core Biopsy Device – Product Description 58
D3Sciences Inc Pipeline Products & Ongoing Clinical Trials Overview 59
Triopsy Edge – Product Status 59
Triopsy Edge – Product Description 59
Dartmouth College Pipeline Products & Ongoing Clinical Trials Overview 60
Electrical Impedance Sensing Biopsy Sampling Device – Product Status 60
Electrical Impedance Sensing Biopsy Sampling Device – Product Description 60
Data Driven Diagnostic Sciences Inc Pipeline Products & Ongoing Clinical Trials Overview 61
Triopsy Edge – Product Status 61
Triopsy Edge – Product Description 61
DEBN Sp zoo Pipeline Products & Ongoing Clinical Trials Overview 62
DEBN (Drug-Eluting Biopsy Needle) – Product Status 62
DEBN (Drug-Eluting Biopsy Needle) – Product Description 62
Delft University of Technology Pipeline Products & Ongoing Clinical Trials Overview 63
System With Shared Control Of Robotic Needle – Product Status 63
System With Shared Control Of Robotic Needle – Product Description 63
Deton Corp. Pipeline Products & Ongoing Clinical Trials Overview 64
Aerosol Biopsy Device – Product Status 64
Aerosol Biopsy Device – Product Description 64
Devicor Medical Products Inc Pipeline Products & Ongoing Clinical Trials Overview 65
HydroMARK Plus Breast Biopsy Site Marker – Product Status 65
HydroMARK Plus Breast Biopsy Site Marker – Product Description 65
Distal Access LLC Pipeline Products & Ongoing Clinical Trials Overview 66
DRIVR Rotational Vacuum-Assisted Biopsy And Tube Cleaning Platform – Product Status 66
DRIVR Rotational Vacuum-Assisted Biopsy And Tube Cleaning Platform – Product Description 66
Duke University Pipeline Products & Ongoing Clinical Trials Overview 67
UGRAB Biopsy Device – Product Status 67
UGRAB Biopsy Device – Product Description 67
Dune Medical Devices Ltd Pipeline Products & Ongoing Clinical Trials Overview 68
Smart Biopsy System – Breast Cancer – Product Status 68
Smart Biopsy System – Breast Cancer – Product Description 68
Ecole Polytechnique Federale de Lausanne Pipeline Products & Ongoing Clinical Trials Overview 69
Transbronchial Biopsy Robotic Catheter – Product Status 69
Transbronchial Biopsy Robotic Catheter – Product Description 69
Emory University Pipeline Products & Ongoing Clinical Trials Overview 70
3D Ultrasound Guided Biopsy System – Product Status 70
3D Ultrasound Guided Biopsy System – Product Description 70
Endocore Ltd Pipeline Products & Ongoing Clinical Trials Overview 71
EndoCore Device – Product Status 71
EndoCore Device – Product Description 71
Endoscopic Assist Devices LLC Pipeline Products & Ongoing Clinical Trials Overview 72
Multiple Biopsy Device – Product Status 72
Multiple Biopsy Device – Product Description 72
EosDx Inc Pipeline Products & Ongoing Clinical Trials Overview 73
Quantum Biopsy Device – Product Status 73
Quantum Biopsy Device – Product Description 73
Fraunhofer-Gesellschaft zur Forderung der Angewandten Forschung eV Pipeline Products & Ongoing Clinical Trials Overview 74
Fine Needle Aspiration Tool – Product Status 74
Fine Needle Aspiration Tool – Product Description 74
Harvard University Pipeline Products & Ongoing Clinical Trials Overview 75
LSS Probe – Pancreatic Cancer – Product Status 75
LSS Probe – Pancreatic Cancer – Product Description 75
iCAD Inc Pipeline Products & Ongoing Clinical Trials Overview 76
MRI-Guided Prostate Biopsy – Product Status 76
MRI-Guided Prostate Biopsy – Product Description 76
Prostate MR/TRUS-Fusion Device – Product Status 77
Prostate MR/TRUS-Fusion Device – Product Description 77
Icahn School of Medicine at Mount Sinai Pipeline Products & Ongoing Clinical Trials Overview 78
Soft Tissue Sampling Tool – Product Status 78
Soft Tissue Sampling Tool – Product Description 78
Indian Institute of Technology Bombay Pipeline Products & Ongoing Clinical Trials Overview 79
Reusable Biopsy Gun – Product Status 79
Reusable Biopsy Gun – Product Description 79
Indio Labs Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 80
BioScoop Biopsy System – Product Status 80
BioScoop Biopsy System – Product Description 80
BxSeal System – Product Status 81
BxSeal System – Product Description 81
Injeq Oy Pipeline Products & Ongoing Clinical Trials Overview 82
INJEQ IQ-Biopsy Needle – Product Status 82
INJEQ IQ-Biopsy Needle – Product Description 82
InLine-Med GmbH Pipeline Products & Ongoing Clinical Trials Overview 83
FlexLine – Product Status 83
FlexLine – Product Description 83
Innobreeze Communication Technologies Private Limited Pipeline Products & Ongoing Clinical Trials Overview 84
Breezescan – Product Status 84
Breezescan – Product Description 84
Breezescan – Cervical Cancer – Product Status 85
Breezescan – Cervical Cancer – Product Description 85
Innovex Medical Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 86
Respiratory Interventional Biopsy Forceps – Product Status 86
Respiratory Interventional Biopsy Forceps – Product Description 86
Single-Use Biopsy Needle – Product Status 87
Single-Use Biopsy Needle – Product Description 87
Single-Use Hot Biopsy Forceps – Product Status 87
Single-Use Hot Biopsy Forceps – Product Description 88
Institute of Cancer Research Pipeline Products & Ongoing Clinical Trials Overview 89
Tissue Microarray Sample Cutter – Product Status 89
Tissue Microarray Sample Cutter – Product Description 89
Intelligent Fiber Optic Systems Corporation Pipeline Products & Ongoing Clinical Trials Overview 90
MEDIFOS – MRI Compatible Needle System – Product Status 90
MEDIFOS – MRI Compatible Needle System – Product Description 90
Johns Hopkins University Pipeline Products & Ongoing Clinical Trials Overview 91
Cryoprobe Capsule – Product Status 91
Cryoprobe Capsule – Product Description 91
Integrated Punch Biopsy Kit – Product Status 92
Integrated Punch Biopsy Kit – Product Description 92
Reusable Biopsy Device – Product Status 92
Reusable Biopsy Device – Product Description 93
Robot-guided Prostate Biopsy Probe – Product Status 93
Robot-guided Prostate Biopsy Probe – Product Description 93
Skin Biopsy Device – Product Status 93
Skin Biopsy Device – Product Description 94
Transrectal Prostate Biopsy Device – Product Status 94
Transrectal Prostate Biopsy Device – Product Description 94
KK Women’s and Children’s Hospital Pipeline Products & Ongoing Clinical Trials Overview 95
7-in-1 Punch Biopsy Device – Product Status 95
7-in-1 Punch Biopsy Device – Product Description 95
Limaca Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 96
Precision GI – Product Status 96
Precision GI – Product Description 96
Massachusetts General Hospital Pipeline Products & Ongoing Clinical Trials Overview 97
IVLCM Capsule – Product Status 97
IVLCM Capsule – Product Description 97
Mayo Clinic Pipeline Products & Ongoing Clinical Trials Overview 98
Full Thickness Gastric Biopsy Needle – Product Status 98
Full Thickness Gastric Biopsy Needle – Product Description 98
Medical Ultrasonics Laboratory Pipeline Products & Ongoing Clinical Trials Overview 99
AdvaNeedle – Product Status 99
AdvaNeedle – Product Description 99
Miniprobes Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 100
Smart Needle – Product Status 100
Smart Needle – Product Description 100
Naviscan Inc Pipeline Products & Ongoing Clinical Trials Overview 101
LumaGUIDE Biopsy Device – Product Status 101
LumaGUIDE Biopsy Device – Product Description 101
NeoDynamics AB Pipeline Products & Ongoing Clinical Trials Overview 102
Cytotest – Product Status 102
Cytotest – Product Description 102
FlexiPulse Needle – Product Status 103
FlexiPulse Needle – Product Description 103
NeoNavia Biopsy System – Product Status 103
NeoNavia Biopsy System – Product Description 104
NeoNavia duo – Product Status 104
NeoNavia duo – Product Description 104
NeoDynamics AB – Ongoing Clinical Trials Overview 105
NeoNavia duo – Evaluation of NeoNavia Biopsy System in Breast Cancer Patients 106
NeoNavia Biopsy System – Comparison Trial of Open-tip Pulsed Needle Biopsy and Conventional Core Biopsy in Axillary Lymph Nodes 107
Omnecoil Instruments Inc Pipeline Products & Ongoing Clinical Trials Overview 108
OmnEcoil – Product Status 108
OmnEcoil – Product Description 108
OnePass Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 109
InstaFan – Fine Needle Aspiration – Product Status 109
InstaFan – Fine Needle Aspiration – Product Description 109
Oregon Health & Science University Pipeline Products & Ongoing Clinical Trials Overview 110
Prostate Biopsy Device – Product Status 110
Prostate Biopsy Device – Product Description 110
Orel State University Pipeline Products & Ongoing Clinical Trials Overview 111
Optical Biopsy System – Product Status 111
Optical Biopsy System – Product Description 111
Physical Sciences Inc Pipeline Products & Ongoing Clinical Trials Overview 112
Smart Multi-grid-guidance Template (SMT) System – Product Status 112
Smart Multi-grid-guidance Template (SMT) System – Product Description 112
Pneutech Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 113
Angled-Path Lung Biopsy Needle System – Product Status 113
Angled-Path Lung Biopsy Needle System – Product Description 113
Precision Biopsy Llc Pipeline Products & Ongoing Clinical Trials Overview 114
ClariCore Biopsy System – Product Status 114
ClariCore Biopsy System – Product Description 114
Prodevice Medical Supplies and Equipment LLC Pipeline Products & Ongoing Clinical Trials Overview 115
Prostate Needle Biopsy Device – Product Status 115
Prostate Needle Biopsy Device – Product Description 115
Promaxo Inc Pipeline Products & Ongoing Clinical Trials Overview 116
PROMAXO MRI Guided Biopsy Device – Product Status 116
PROMAXO MRI Guided Biopsy Device – Product Description 116
Quality ElectroDynamics LLC Pipeline Products & Ongoing Clinical Trials Overview 117
RF Breast-Coil-Biopsy-Plate System – Product Status 117
RF Breast-Coil-Biopsy-Plate System – Product Description 117
Queen Mary University of London Pipeline Products & Ongoing Clinical Trials Overview 118
3D MRI-US Image Fusion System – Product Status 118
3D MRI-US Image Fusion System – Product Description 118
Rad/Path Solutions LLC Pipeline Products & Ongoing Clinical Trials Overview 119
Pathology Tissue Tray – Product Status 119
Pathology Tissue Tray – Product Description 119
ReCor Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 120
Reusable Biopsy System – Product Status 120
Reusable Biopsy System – Product Description 120
Resitu Medical AB Pipeline Products & Ongoing Clinical Trials Overview 121
Resitu Instrument – Product Status 121
Resitu Instrument – Product Description 121
Robin Medical, Inc. Pipeline Products & Ongoing Clinical Trials Overview 122
Vacuum-Assisted Biopsy Device – Product Status 122
Vacuum-Assisted Biopsy Device – Product Description 122
Robopsy Pipeline Products & Ongoing Clinical Trials Overview 123
Robopsy System – Product Status 123
Robopsy System – Product Description 123
Rutgers The State University of New Jersey Pipeline Products & Ongoing Clinical Trials Overview 124
Vascular Access Needle Assembly – Product Status 124
Vascular Access Needle Assembly – Product Description 124
Saint Joseph Medical Center Pipeline Products & Ongoing Clinical Trials Overview 125
Clear Biopsy Device – Product Status 125
Clear Biopsy Device – Product Description 125
Sanovas Inc Pipeline Products & Ongoing Clinical Trials Overview 126
Transbronchial Pressure Vacuum Actuated (TPVA) Micro Biopsy Capsule – Product Status 126
Transbronchial Pressure Vacuum Actuated (TPVA) Micro Biopsy Capsule – Product Description 126
SpectraScience Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 127
Next Generation Multi-Modal Fluorescence And Broadband Spectroscopy System – Product Status 127
Next Generation Multi-Modal Fluorescence And Broadband Spectroscopy System – Product Description 127
WavSTAT – Pancreatic Cancer – Product Status 128
WavSTAT – Pancreatic Cancer – Product Description 128
WavSTAT Optial Biopsy System – Inflammatory Bowel Syndrome – Product Status 128
WavSTAT Optial Biopsy System – Inflammatory Bowel Syndrome – Product Description 129
WavSTAT Optial Biopsy System – Lung Cancer – Product Status 129
WavSTAT Optial Biopsy System – Lung Cancer – Product Description 129
WavSTAT Optical Biopsy System – Esophageal Cancer – Product Status 130
WavSTAT Optical Biopsy System – Esophageal Cancer – Product Description 130
TeesuVac ApS Pipeline Products & Ongoing Clinical Trials Overview 131
Advanced Biopsy Gun – Product Status 131
Advanced Biopsy Gun – Product Description 131
Biopsy Gun – Product Status 132
Biopsy Gun – Product Description 132
The Lundquist Institute Pipeline Products & Ongoing Clinical Trials Overview 133
Needle Guidance Device – Product Status 133
Needle Guidance Device – Product Description 133
Tokyo Institute of Technology Pipeline Products & Ongoing Clinical Trials Overview 134
Self-Propelled Catheter – Product Status 134
Self-Propelled Catheter – Product Description 134
Trajan Scientific Australia Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 135
Microbiopsy Device – Product Status 135
Microbiopsy Device – Product Description 135
TransMed7 LLC Pipeline Products & Ongoing Clinical Trials Overview 136
Heron – 16G – Product Status 136
Heron – 16G – Product Description 137
Heron – 18G – Product Status 137
Heron – 18G – Product Description 137
Heron XPS – Product Status 138
Heron XPS – Product Description 138
Martinet Soft Tissue Excisional Biopsy Device – Product Status 138
Martinet Soft Tissue Excisional Biopsy Device – Product Description 139
Next-Gen Cardinal Fine-Needle Core Biopsy Device – Product Status 139
Next-Gen Cardinal Fine-Needle Core Biopsy Device – Product Description 139
Osprey – Product Status 140
Osprey – Product Description 140
Phoenix Endoscopic Biopsy Device – Product Status 140
Phoenix Endoscopic Biopsy Device – Product Description 141
Thunderbird – Product Status 141
Thunderbird – Product Description 141
Triopsy Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 142
3D Biopsy Mapping System – Product Status 142
3D Biopsy Mapping System – Product Description 143
3D Biopsy System – Breast Cancer – Product Status 143
3D Biopsy System – Breast Cancer – Product Description 143
3D Biopsy System – Kidney Cancer – Product Status 144
3D Biopsy System – Kidney Cancer – Product Description 144
3D Biopsy System – Liver Cancer – Product Status 144
3D Biopsy System – Liver Cancer – Product Description 145
3D Biopsy System – Thyroid Cancer – Product Status 145
3D Biopsy System – Thyroid Cancer – Product Description 145
Biopsy Device – Product Status 146
Biopsy Device – Product Description 146
Biopsy Needle – Product Status 146
Biopsy Needle – Product Description 146
Triopsy Integrated Pathology System – Product Status 147
Triopsy Integrated Pathology System – Product Description 147
Tufts University Pipeline Products & Ongoing Clinical Trials Overview 148
INFORCE Biopsy Device – Product Status 148
INFORCE Biopsy Device – Product Description 148
University of California San Diego Pipeline Products & Ongoing Clinical Trials Overview 149
Muscle Biopsy Clamp – Product Status 149
Muscle Biopsy Clamp – Product Description 149
University of California San Francisco Pipeline Products & Ongoing Clinical Trials Overview 150
Intraprocedural Grid Localization System – Product Status 150
Intraprocedural Grid Localization System – Product Description 150
Safe Endovascular Biospy Device – Product Status 151
Safe Endovascular Biospy Device – Product Description 151
University of Cambridge Pipeline Products & Ongoing Clinical Trials Overview 152
CAMPROBE – Product Status 152
CAMPROBE – Product Description 152
University of Cincinnati Pipeline Products & Ongoing Clinical Trials Overview 153
Syringe Based Rapid Processor – Product Status 153
Syringe Based Rapid Processor – Product Description 153
University of Florida Pipeline Products & Ongoing Clinical Trials Overview 154
Coaxial Biopsy Needle – Product Status 154
Coaxial Biopsy Needle – Product Description 154
University of Illinois at Urbana-Champaign Pipeline Products & Ongoing Clinical Trials Overview 155
OCT Enhanced Biopsy Needle – Product Status 155
OCT Enhanced Biopsy Needle – Product Description 155
University of Louisville Pipeline Products & Ongoing Clinical Trials Overview 156
Suction Assisted Multiple Biopsy Device – Product Status 156
Suction Assisted Multiple Biopsy Device – Product Description 156
University of Michigan Pipeline Products & Ongoing Clinical Trials Overview 157
Fine Aspiration Biopsy Needle – Product Status 157
Fine Aspiration Biopsy Needle – Product Description 157
Renal Biopsy Device – Product Status 158
Renal Biopsy Device – Product Description 158
Skin Tissue Biopsy Device – Product Status 158
Skin Tissue Biopsy Device – Product Description 159
University of Minnesota Pipeline Products & Ongoing Clinical Trials Overview 160
Electrical Impedance Epidural Needle – Product Status 160
Electrical Impedance Epidural Needle – Product Description 160
Intracranial Endoscopy System – Product Status 161
Intracranial Endoscopy System – Product Description 161
Lung Biopsy Tool – Product Status 161
Lung Biopsy Tool – Product Description 162
Multi-Compartment Biopsy Syringe – Product Status 162
Multi-Compartment Biopsy Syringe – Product Description 162
University of Missouri Pipeline Products & Ongoing Clinical Trials Overview 163
Needle Aspiration Device – Product Status 163
Needle Aspiration Device – Product Description 163
University of Queensland Pipeline Products & Ongoing Clinical Trials Overview 164
Micropunch – Product Status 164
Micropunch – Product Description 164
University of Texas Health Science Center at San Antonio Pipeline Products & Ongoing Clinical Trials Overview 165
Mediastinal Needle Biopsy Device – Product Status 165
Mediastinal Needle Biopsy Device – Product Description 165
University of Twente Pipeline Products & Ongoing Clinical Trials Overview 166
Sunram 5 – Product Status 166
Sunram 5 – Product Description 166
University of Washington Pipeline Products & Ongoing Clinical Trials Overview 167
Endometrial Biopsy Device – Product Status 167
Endometrial Biopsy Device – Product Description 167
Vanderbilt University Pipeline Products & Ongoing Clinical Trials Overview 168
Core and Side Cut Biopsy Device – Product Status 168
Core and Side Cut Biopsy Device – Product Description 168
Steerable Robotic Needle – Lung Biopsy – Product Status 169
Steerable Robotic Needle – Lung Biopsy – Product Description 169
Vascular BioSciences Pipeline Products & Ongoing Clinical Trials Overview 170
EABx – COVID-19 – Product Status 170
EABx – COVID-19 – Product Description 170
EABx – Lung Transplant Rejection – Product Status 171
EABx – Lung Transplant Rejection – Product Description 171
EABx – Pulmonary Arterial Hypertension – Product Status 171
EABx – Pulmonary Arterial Hypertension – Product Description 172
Verisante Technology Inc Pipeline Products & Ongoing Clinical Trials Overview 173
Verisante Core – Cervical Cancer – Product Status 173
Verisante Core – Cervical Cancer – Product Description 173
Verisante Core – Gastrointestinal Cancer – Product Status 174
Verisante Core – Gastrointestinal Cancer – Product Description 174
Yale University Pipeline Products & Ongoing Clinical Trials Overview 175
Painless Bone Biopsy Needle – Product Status 175
Painless Bone Biopsy Needle – Product Description 175
Zamar Care Pipeline Products & Ongoing Clinical Trials Overview 176
Evocut Needle – Product Status 176
Evocut Needle – Product Description 176
Glossary 220
![]()