|1 Table of Contents 3
|1.1 List of Tables 10
|1.2 List of Figures 18
2 Introduction 19
2.1 Cardiac Catheters Overview 19
3 Products under Development 20
3.1 Cardiac Catheters – Pipeline Products by Stage of Development 20
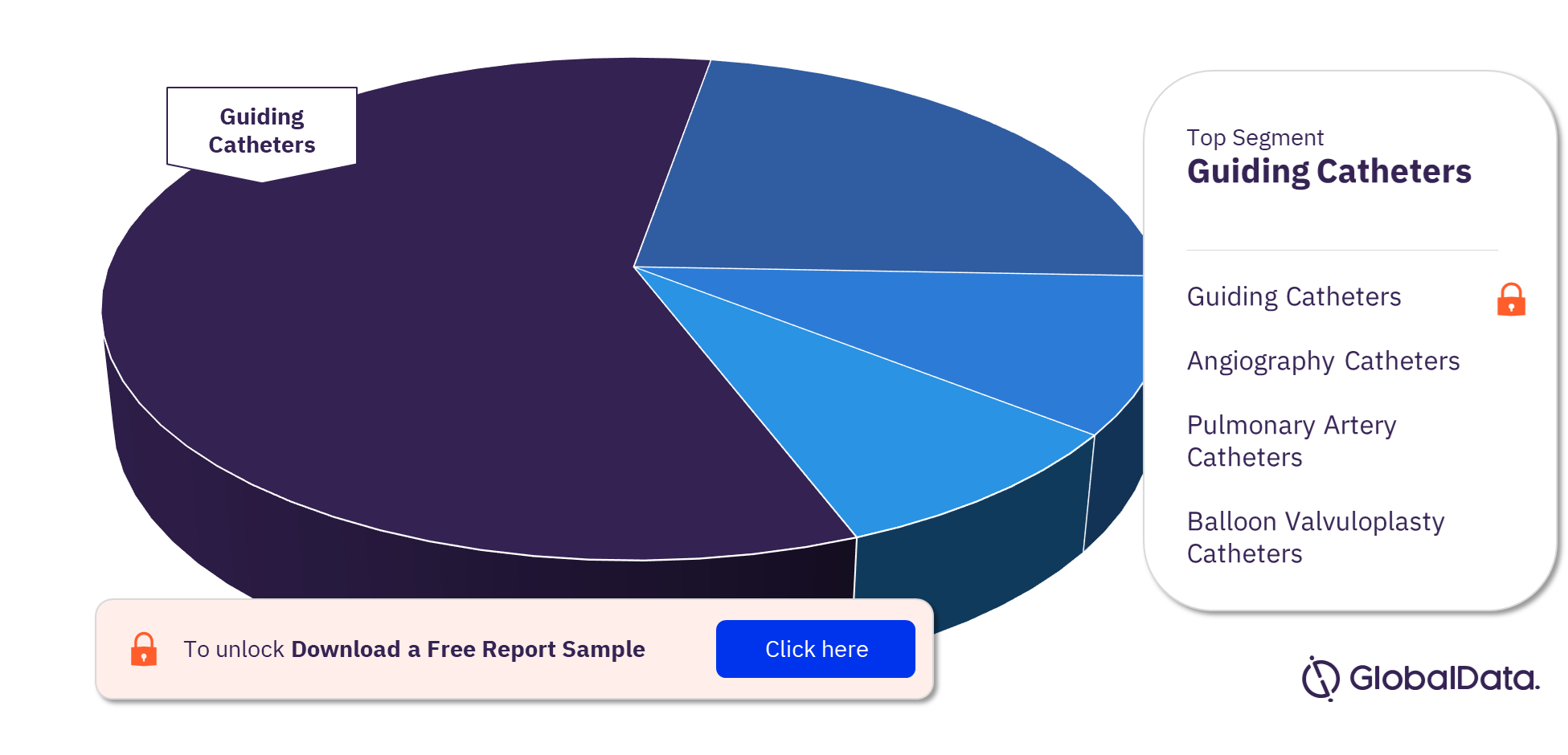
3.2 Cardiac Catheters – Pipeline Products by Segment 21
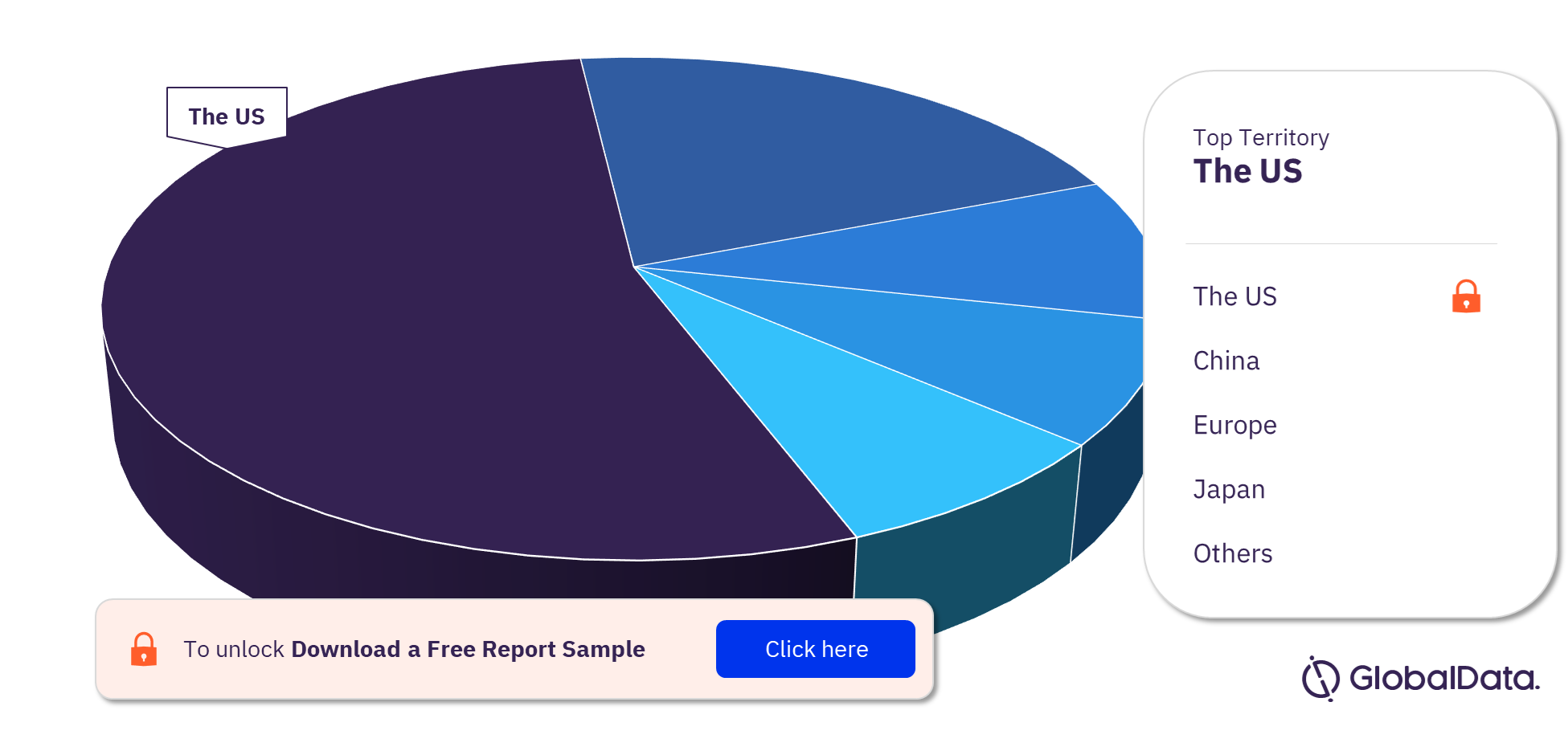
3.3 Cardiac Catheters – Pipeline Products by Territory 22
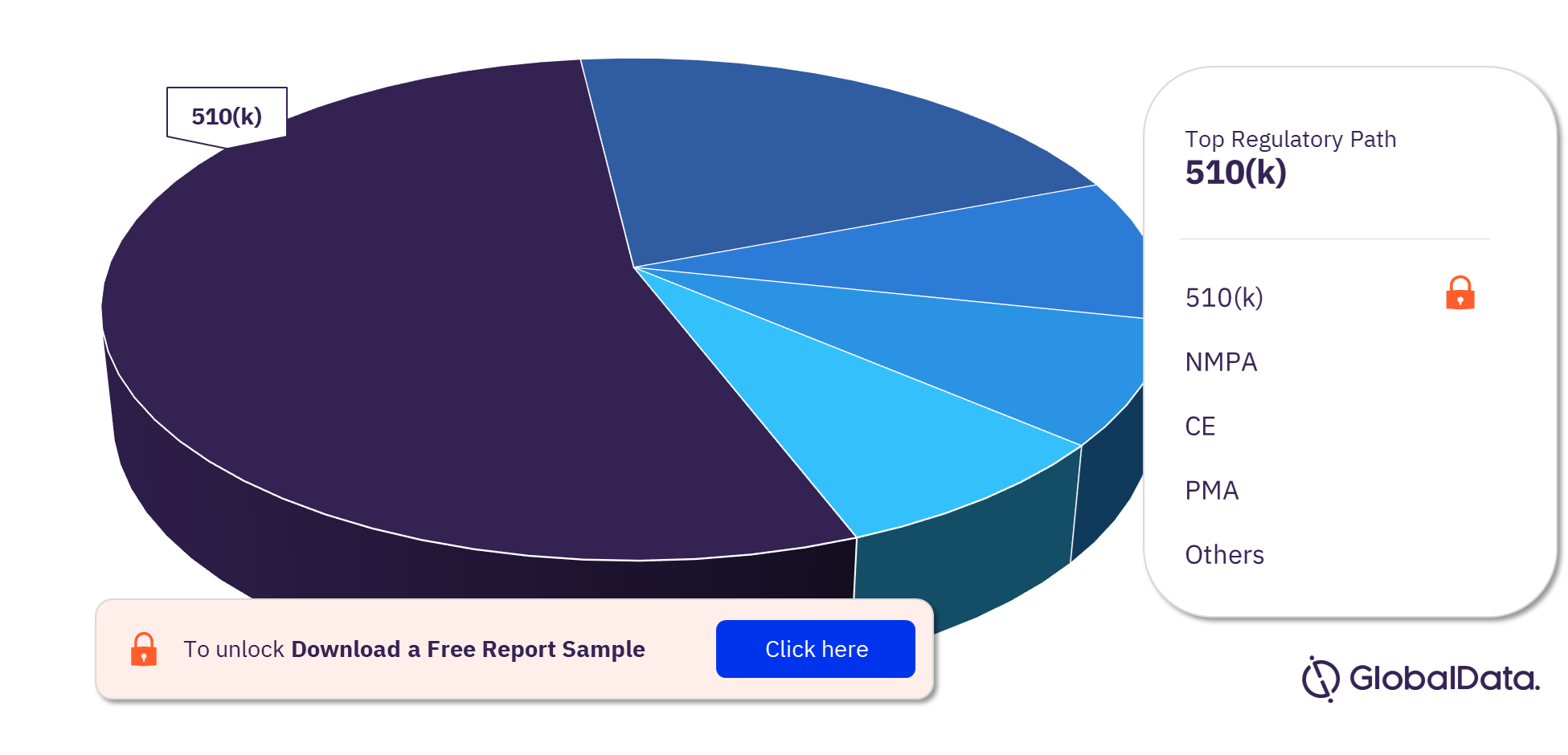
3.4 Cardiac Catheters – Pipeline Products by Regulatory Path 23
3.5 Cardiac Catheters – Pipeline Products by Estimated Approval Date 24
3.6 Cardiac Catheters – Ongoing Clinical Trials 25
4 Cardiac Catheters – Pipeline Products under Development by Companies 26
4.1 Cardiac Catheters Companies – Pipeline Products by Stage of Development 26
4.2 Cardiac Catheters – Pipeline Products by Stage of Development 30
5 Cardiac Catheters Companies and Product Overview 34
5.1 3DT Holdings LLC Company Overview 34
5.1.1 3DT Holdings LLC Pipeline Products & Ongoing Clinical Trials Overview 34
5.2 Achieva Medical (Shanghai) Co Ltd Company Overview 37
5.2.1 Achieva Medical (Shanghai) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 37
5.3 Acotec Scientific Holdings Ltd Company Overview 38
5.3.1 Acotec Scientific Holdings Ltd Pipeline Products & Ongoing Clinical Trials Overview 38
5.4 Affera Inc Company Overview 41
5.4.1 Affera Inc Pipeline Products & Ongoing Clinical Trials Overview 41
5.5 AlviMedica Medical Technologies Inc Company Overview 42
5.5.1 AlviMedica Medical Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 42
5.6 AngioDynamics Inc Company Overview 44
5.6.1 AngioDynamics Inc Pipeline Products & Ongoing Clinical Trials Overview 44
5.7 APT Medical Inc Company Overview 45
5.7.1 APT Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 45
5.8 Aptorum Group Ltd Company Overview 48
5.8.1 Aptorum Group Ltd Pipeline Products & Ongoing Clinical Trials Overview 48
5.9 Avantec Vascular Corp Company Overview 49
5.9.1 Avantec Vascular Corp Pipeline Products & Ongoing Clinical Trials Overview 49
5.10 Avinger Inc Company Overview 50
5.10.1 Avinger Inc Pipeline Products & Ongoing Clinical Trials Overview 50
5.11 Balch Hill Medical Group Company Overview 52
5.11.1 Balch Hill Medical Group Pipeline Products & Ongoing Clinical Trials Overview 52
5.12 Bendit Technologies Ltd Company Overview 53
5.12.1 Bendit Technologies Ltd Pipeline Products & Ongoing Clinical Trials Overview 53
5.13 Biosensors International Group Ltd Company Overview 55
5.13.1 Biosensors International Group Ltd Pipeline Products & Ongoing Clinical Trials Overview 55
5.14 Bioteque Corp Company Overview 56
5.14.1 Bioteque Corp Pipeline Products & Ongoing Clinical Trials Overview 56
5.15 Boston Children’s Hospital Company Overview 57
5.15.1 Boston Children’s Hospital Pipeline Products & Ongoing Clinical Trials Overview 57
5.16 Boston Scientific Corp Company Overview 58
5.16.1 Boston Scientific Corp Pipeline Products & Ongoing Clinical Trials Overview 58
5.17 Boston TransTec LLC Company Overview 60
5.17.1 Boston TransTec LLC Pipeline Products & Ongoing Clinical Trials Overview 60
5.18 Boston University Company Overview 61
5.18.1 Boston University Pipeline Products & Ongoing Clinical Trials Overview 61
5.19 Cardiovascular Systems Inc Company Overview 62
5.19.1 Cardiovascular Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 62
5.20 Cook Medical Inc Company Overview 65
5.20.1 Cook Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 65
5.21 Cordis Corp Company Overview 68
5.21.1 Cordis Corp Pipeline Products & Ongoing Clinical Trials Overview 68
5.22 CoreMedic AG Company Overview 69
5.22.1 CoreMedic AG Pipeline Products & Ongoing Clinical Trials Overview 69
5.23 Crossliner Inc Company Overview 70
5.23.1 Crossliner Inc Pipeline Products & Ongoing Clinical Trials Overview 70
5.24 Delft University of Technology Company Overview 71
5.24.1 Delft University of Technology Pipeline Products & Ongoing Clinical Trials Overview 71
5.25 Deliverance Ltd. Company Overview 72
5.25.1 Deliverance Ltd. Pipeline Products & Ongoing Clinical Trials Overview 72
5.26 Echopoint Medical Ltd Company Overview 73
5.26.1 Echopoint Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 73
5.27 Emory University Company Overview 76
5.27.1 Emory University Pipeline Products & Ongoing Clinical Trials Overview 76
5.28 EP Ltd Company Overview 77
5.28.1 EP Ltd Pipeline Products & Ongoing Clinical Trials Overview 77
5.29 Flow MedTech LLC Company Overview 78
5.29.1 Flow MedTech LLC Pipeline Products & Ongoing Clinical Trials Overview 78
5.30 Fraunhofer Institute for Medical Image Computing MEVIS Company Overview 79
5.30.1 Fraunhofer Institute for Medical Image Computing MEVIS Pipeline Products & Ongoing Clinical Trials Overview 79
5.31 Georgia Institute of Technology Company Overview 80
5.31.1 Georgia Institute of Technology Pipeline Products & Ongoing Clinical Trials Overview 80
5.32 Hadasit Medical Research Services and Development Ltd Company Overview 81
5.32.1 Hadasit Medical Research Services and Development Ltd Pipeline Products & Ongoing Clinical Trials Overview 81
5.33 Harvard University Company Overview 82
5.33.1 Harvard University Pipeline Products & Ongoing Clinical Trials Overview 82
5.34 HemoCath Ltd Company Overview 83
5.34.1 HemoCath Ltd Pipeline Products & Ongoing Clinical Trials Overview 83
5.35 HoliStick Medical Company Overview 84
5.35.1 HoliStick Medical Pipeline Products & Ongoing Clinical Trials Overview 84
5.36 Innoveta Biomedical LLC Company Overview 85
5.36.1 Innoveta Biomedical LLC Pipeline Products & Ongoing Clinical Trials Overview 85
5.37 Innovia LLC Company Overview 86
5.37.1 Innovia LLC Pipeline Products & Ongoing Clinical Trials Overview 86
5.38 Insight Lifetech Co Ltd Company Overview 87
5.38.1 Insight Lifetech Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 87
5.39 InterShunt Technologies Inc Company Overview 88
5.39.1 InterShunt Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 88
5.40 Intratech Medical Ltd. Company Overview 91
5.40.1 Intratech Medical Ltd. Pipeline Products & Ongoing Clinical Trials Overview 91
5.41 iVascular SLU Company Overview 94
5.41.1 iVascular SLU Pipeline Products & Ongoing Clinical Trials Overview 94
5.42 Jiangsu Zhenyi Medical Technology Co Ltd Company Overview 97
5.42.1 Jiangsu Zhenyi Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 97
5.43 Johns Hopkins University Company Overview 98
5.43.1 Johns Hopkins University Pipeline Products & Ongoing Clinical Trials Overview 98
5.44 Kaminari Medical BV Company Overview 99
5.44.1 Kaminari Medical BV Pipeline Products & Ongoing Clinical Trials Overview 99
5.45 Kaneka Corp Company Overview 100
5.45.1 Kaneka Corp Pipeline Products & Ongoing Clinical Trials Overview 100
5.46 Leibniz Institute of Photonic Technology Company Overview 102
5.46.1 Leibniz Institute of Photonic Technology Pipeline Products & Ongoing Clinical Trials Overview 102
5.47 Magneto Thrombectomy Solutions Ltd Company Overview 103
5.47.1 Magneto Thrombectomy Solutions Ltd Pipeline Products & Ongoing Clinical Trials Overview 103
5.48 MaRVis Technologies GmbH Company Overview 104
5.48.1 MaRVis Technologies GmbH Pipeline Products & Ongoing Clinical Trials Overview 104
5.49 Mayo Clinic Company Overview 105
5.49.1 Mayo Clinic Pipeline Products & Ongoing Clinical Trials Overview 105
5.50 MC3 Inc Company Overview 107
5.50.1 MC3 Inc Pipeline Products & Ongoing Clinical Trials Overview 107
5.51 Medical Device Investments, Inc. Company Overview 108
5.51.1 Medical Device Investments, Inc. Pipeline Products & Ongoing Clinical Trials Overview 108
5.52 Medtronic Plc Company Overview 110
5.52.1 Medtronic Plc Pipeline Products & Ongoing Clinical Trials Overview 110
5.53 Merit Medical Systems Inc Company Overview 112
5.53.1 Merit Medical Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 112
5.54 MicroPort Scientific Corp Company Overview 113
5.54.1 MicroPort Scientific Corp Pipeline Products & Ongoing Clinical Trials Overview 113
5.55 Minnesota Health Solutions Corporation Company Overview 115
5.55.1 Minnesota Health Solutions Corporation Pipeline Products & Ongoing Clinical Trials Overview 115
5.56 Moray Medical Company Overview 116
5.56.1 Moray Medical Pipeline Products & Ongoing Clinical Trials Overview 116
5.57 Myocardial Assist Systems and Technology LLC Company Overview 117
5.57.1 Myocardial Assist Systems and Technology LLC Pipeline Products & Ongoing Clinical Trials Overview 117
5.58 NeuroInterventional Therapeutics, Inc. Company Overview 118
5.58.1 NeuroInterventional Therapeutics, Inc. Pipeline Products & Ongoing Clinical Trials Overview 118
5.59 NeuroVasc Technologies Inc Company Overview 119
5.59.1 NeuroVasc Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 119
5.60 NewMed Medical Co Ltd Company Overview 120
5.60.1 NewMed Medical Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 120
5.61 Nipro Corp Company Overview 122
5.61.1 Nipro Corp Pipeline Products & Ongoing Clinical Trials Overview 122
5.62 Northwestern University Company Overview 123
5.62.1 Northwestern University Pipeline Products & Ongoing Clinical Trials Overview 123
5.63 Ohio State University Wexner Medical Center Company Overview 124
5.63.1 Ohio State University Wexner Medical Center Pipeline Products & Ongoing Clinical Trials Overview 124
5.64 OrbusNeich Company Overview 125
5.64.1 OrbusNeich Pipeline Products & Ongoing Clinical Trials Overview 125
5.65 Oxus Medical Inc Company Overview 128
5.65.1 Oxus Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 128
5.66 Peijia Medical Ltd Company Overview 129
5.66.1 Peijia Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 129
5.67 Perseus Biomed Ltd Company Overview 133
5.67.1 Perseus Biomed Ltd Pipeline Products & Ongoing Clinical Trials Overview 133
5.68 Pi-Cardia Ltd Company Overview 134
5.68.1 Pi-Cardia Ltd Pipeline Products & Ongoing Clinical Trials Overview 134
5.69 Pulnovo Medical (Wuxi) Co Ltd Company Overview 138
5.69.1 Pulnovo Medical (Wuxi) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 138
5.70 QuantumCor Inc. (Inactive) Company Overview 143
5.70.1 QuantumCor Inc. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 143
5.71 Ra Medical Systems Inc Company Overview 144
5.71.1 Ra Medical Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 144
5.72 ReCor Medical Inc Company Overview 145
5.72.1 ReCor Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 145
5.73 Revamp Medical Ltd Company Overview 146
5.73.1 Revamp Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 146
5.74 Rutgers The State University of New Jersey Company Overview 149
5.74.1 Rutgers The State University of New Jersey Pipeline Products & Ongoing Clinical Trials Overview 149
5.75 Shanghai Changde Medical Technology Co Ltd Company Overview 150
5.75.1 Shanghai Changde Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 150
5.76 Shanghai Healing Medical Instruments Co Ltd Company Overview 152
5.76.1 Shanghai Healing Medical Instruments Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 152
5.77 Shockwave Medical Inc Company Overview 153
5.77.1 Shockwave Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 153
5.78 SkyDance Vascular Inc Company Overview 154
5.78.1 SkyDance Vascular Inc Pipeline Products & Ongoing Clinical Trials Overview 154
5.79 Sonix Therapeutics Company Overview 155
5.79.1 Sonix Therapeutics Pipeline Products & Ongoing Clinical Trials Overview 155
5.80 SoundBite Medical Solutions Inc Company Overview 156
5.80.1 SoundBite Medical Solutions Inc Pipeline Products & Ongoing Clinical Trials Overview 156
5.81 SpectraWAVE Inc Company Overview 157
5.81.1 SpectraWAVE Inc Pipeline Products & Ongoing Clinical Trials Overview 157
5.82 Spectrumedics Medical Technology (Shanghai) Co Ltd Company Overview 160
5.82.1 Spectrumedics Medical Technology (Shanghai) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 160
5.83 Stelect Pty Ltd Company Overview 161
5.83.1 Stelect Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 161
5.84 Strait Access Technologies (Pty) Ltd Company Overview 162
5.84.1 Strait Access Technologies (Pty) Ltd Pipeline Products & Ongoing Clinical Trials Overview 162
5.85 SurModics Inc Company Overview 163
5.85.1 SurModics Inc Pipeline Products & Ongoing Clinical Trials Overview 163
5.86 Therix Medical, Inc. (Inactive) Company Overview 164
5.86.1 Therix Medical, Inc. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 164
5.87 Three Rivers Cardiovascular Systems Inc Company Overview 165
5.87.1 Three Rivers Cardiovascular Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 165
5.88 TransCardiac Therapeutics, LLC Company Overview 166
5.88.1 TransCardiac Therapeutics, LLC Pipeline Products & Ongoing Clinical Trials Overview 166
5.89 U.S. Department of Veterans Affairs Company Overview 167
5.89.1 U.S. Department of Veterans Affairs Pipeline Products & Ongoing Clinical Trials Overview 167
5.90 U.S. Stem Cell Inc Company Overview 168
5.90.1 U.S. Stem Cell Inc Pipeline Products & Ongoing Clinical Trials Overview 168
5.91 University of Adelaide Company Overview 169
5.91.1 University of Adelaide Pipeline Products & Ongoing Clinical Trials Overview 169
5.92 University of California Davis Company Overview 170
5.92.1 University of California Davis Pipeline Products & Ongoing Clinical Trials Overview 170
5.93 University of California Los Angeles Company Overview 171
5.93.1 University of California Los Angeles Pipeline Products & Ongoing Clinical Trials Overview 171
5.94 University of California San Francisco Company Overview 172
5.94.1 University of California San Francisco Pipeline Products & Ongoing Clinical Trials Overview 172
5.95 University of Chicago Company Overview 174
5.95.1 University of Chicago Pipeline Products & Ongoing Clinical Trials Overview 174
5.96 University of Leicester Company Overview 175
5.96.1 University of Leicester Pipeline Products & Ongoing Clinical Trials Overview 175
5.97 University of Limerick Company Overview 176
5.97.1 University of Limerick Pipeline Products & Ongoing Clinical Trials Overview 176
5.98 University of Montreal Company Overview 177
5.98.1 University of Montreal Pipeline Products & Ongoing Clinical Trials Overview 177
5.99 University of Nebraska Company Overview 178
5.99.1 University of Nebraska Pipeline Products & Ongoing Clinical Trials Overview 178
5.100 Vanderbilt University Company Overview 179
5.100.1 Vanderbilt University Pipeline Products & Ongoing Clinical Trials Overview 179
5.101 VerAvanti Inc Company Overview 180
5.101.1 VerAvanti Inc Pipeline Products & Ongoing Clinical Trials Overview 180
5.102 Voyage Medical (Inactive) Company Overview 181
5.102.1 Voyage Medical (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 181
6 Cardiac Catheters- Recent Developments 182
6.1 Jan 10, 2023: Inari Medical Announces Preliminary 2022 Revenue and 2023 Guidance 182
6.2 Jan 10, 2023: Vygon Group appoints Ludovic Richard-Vitton as CEO 183
6.3 Dec 13, 2022: Johnson & Johnson to Host Investor Conference Call on Fourth-Quarter Results 183
6.4 Dec 08, 2022: Edwards Lifesciences Announces CEO Succession Plan 183
6.5 Dec 07, 2022: Cook Medical welcomes Dr. John A. Kaufman as Chief Medical Officer 184
6.6 Dec 07, 2022: Johnson & Johnson to Participate in the 41st Annual J.P. Morgan Healthcare Conference 185
6.7 Nov 23, 2022: Medtronic Reports Quarterly Earnings 185
6.8 Nov 22, 2022: Medtronic reports second quarter fiscal 2023 financial results 185
6.9 Nov 22, 2022: Mayo Clinic surgeon takes the wheel as Kwik Trip’s new CEO 191
6.10 Nov 15, 2022: Medtronic Set to Announce Earnings on Tuesday 191
6.11 Nov 15, 2022: MySize Reports Third Quarter Financial Results 191
6.12 Nov 11, 2022: Asahi Intecc Consolidated Financial Results for the Three Months Ended September 30, 2022 193
6.13 Nov 10, 2022: Terumo Corp Announces Consolidated Financial Results for the Second Quarter Ended September 30, 2022 194
6.14 Nov 04, 2022: JMS Co. Announces Consolidated Financial Highlights for the Interim Period Ended September 2022 194
6.15 Nov 02, 2022: Inari Medical Reports Third Quarter 2022 Financial Results 195
6.16 Nov 01, 2022: Abiomed Announces Second Quarter Revenue of $266 Million, up 11% in Constant Currency, up 7% on a Reported Basis Year Over Year 196
6.17 Nov 01, 2022: New randomized controlled study published in JAMA demonstrates the value of clinical decision support tools in preventing acute kidney injury in patients undergoing coronary angiography 198
6.18 Oct 27, 2022: Teleflex Reports Third Quarter Financial Results And Full Year Outlook 198
6.19 Oct 25, 2022: General Electric Announces third-quarter results 199
6.20 Oct 20, 2022: Edwards Lifesciences to Host Earnings Conference Call on October 27, 2022 200
6.21 Oct 18, 2022: Johnson & Johnson Reports Q3 2022 Results 200
6.22 Oct 17, 2022: LeMaitre Will Announce Third Quarter 2022 Earnings Results October 27, 2022 202
6.23 Oct 13, 2022: Teleflex Announces Third Quarter 2022 Earnings Conference Call Information 202
6.24 Oct 13, 2022: Merit Medical Systems to Announce Third Quarter Results on October 26, 2022 203
6.25 Oct 12, 2022: Cardiovascular Systems to Host Earnings Conference Call on November 3, 2022 203
6.26 Oct 06, 2022: Inari Medical to Announce Third Quarter 2022 Financial Results 203
6.27 Oct 06, 2022: General Electric starts laying off workers at onshore wind business unit 203
6.28 Sep 30, 2022: Natalie Caine named chief administrative officer at Mayo Clinic in Rochester 204
6.29 Sep 23, 2022: Getinge appoints Joanna Engelke as new Executive Vice President Quality Compliance, Regulatory & Medical Affairs and member of the Getinge Executive Team 204
6.30 Sep 21, 2022: General Electric appoints Repsol’s Zingoni as CEO of power business 204
6.31 Sep 21, 2022: Rainmed Medical Ltd announced interim report for 2022 204
6.32 Sep 13, 2022: Johnson & Johnson to Host Investor Conference Call on Third-Quarter Results 205
6.33 Sep 13, 2022: TCT 2022: Impella enables complete revascularization, improved quality of life and native heart recovery 205
6.34 Sep 07, 2022: Baxter to Host Third-Quarter 2022 Financial Results Conference Call for Investors 206
6.35 Sep 07, 2022: Medtronic to appear at September investor conferences 207
6.36 Aug 31, 2022: LeMaitre to Present at the Morgan Stanley 20th Annual Global Healthcare Conference 207
6.37 Aug 25, 2022: Atrion Announces CFO Succession Plan 207
6.38 Aug 24, 2022: Johnson & Johnson Appoints Larry Merlo as Non-Executive Chair Designate of Planned New Consumer Health Company 207
6.39 Aug 24, 2022: JNJ Appoints Merlo as Non-Executive Chair Designate 208
6.40 Aug 23, 2022: Medtronic reports first quarter fiscal 2023 financial results 208
6.41 Aug 23, 2022: Medtronic Announces Quarterly Earnings Results 211
6.42 Aug 16, 2022: Boston Scientific Announces Upcoming Conference Schedule 214
6.43 Aug 11, 2022: Neovasc Announces Second Quarter Financial Results and Provides Corporate Update 214
6.44 Aug 10, 2022: Medline releases 2021 Corporate Social Responsibility Report 215
6.45 Aug 09, 2022: Johnson & Johnson to Participate in the Morgan Stanley 20th Annual Global Healthcare Conference 216
6.46 Aug 05, 2022: JMS Co. Announces Consolidated Financial Highlights for the Interim Period Ended June 2022 216
6.47 Aug 04, 2022: ABIOMED Announces First Quarter Record Revenue of $277 Million, up 10% Year Over Year, up 12% in Constant Currency 216
6.48 Aug 03, 2022: Inari Medical Announces Leadership Succession Plan 219
6.49 Aug 03, 2022: Inari Medical Reports Second Quarter 2022 Financial Results 220
6.50 Aug 02, 2022: Johnson & Johnson to Participate in the 2022 Wells Fargo Securities Healthcare Conference 221
6.51 Jul 28, 2022: LeMaitre Q2 2022 Financial Results 221
6.52 Jul 27, 2022: Merit Medical Reports Results for Quarter Ended June 30, 2022 222
6.53 Jul 26, 2022: UTMD Reports Financial Performance for Second Calendar Quarter and First Half 2022 223
6.54 Jul 24, 2022: Annapolis Financial Services Has $359,000 Stake in Johnson & Johnson 229
6.55 Jul 21, 2022: Edwards Lifesciences to Host Earnings Conference Call on July 28, 2022 229
6.56 Jul 20, 2022: Johnson & Johnson cuts sales forecasts 229
6.57 Jul 20, 2022: Abbott Reports Second-Quarter 2022 Results and Raises Full-Year EPS Guidance 229
6.58 Jul 18, 2022: Johnson & Johnson’s Executive Vice President, General Counsel Michael Ullmann to Retire; Elizabeth Forminard Named General Counsel, Effective October 17, 2022 230
6.59 Jul 18, 2022: LeMaitre Will Announce Second Quarter 2022 Earnings Results July 28, 2022 231
6.60 Jul 18, 2022: Inari Medical to Announce Second Quarter 2022 Financial Results 231
6.61 Jul 14, 2022: Merit Medical Systems to Announce Second Quarter Results on July 27, 2022 231
6.62 Jul 14, 2022: Abiomed First Quarter Fiscal 2023 Earnings and Conference Call Notification 232
6.63 Jul 11, 2022: Rainmed nets $19M in Hong Kong IPO debut 232
6.64 Jul 08, 2022: Cordis Names Bryan Loo President of Asia-pacific Region 232
6.65 Jul 04, 2022: Getinge announces its Q2 Report 2022 on July 19 at 08:00 CEST followed by a conference call at 10:00 CEST 232
6.66 Jul 01, 2022: Osprey Medical : Business restructure 233
6.67 Jun 28, 2022: Japan Lifeline Co. Notice of Resolution of the 42nd Annual General Meeting of Shareholders 233
6.68 Jun 28, 2022: Nipro Notice of Resolutions at the 69th Annual General Meeting of Shareholders 234
6.69 Jun 27, 2022: Medtronic Board appoints Lidia Fonseca as a new director 235
6.70 Jun 08, 2022: Baxter to Host Second-Quarter 2022 Financial Results Conference Call for Investors 236
6.71 Jun 07, 2022: Teleflex launches new arrow pressure injectable midline catheter in Europe, the Middle East and Africa region 236
6.72 Jun 07, 2022: Cardiovascular Systems chief operating officer Rhonda Robb exits company 236
6.73 May 27, 2022: Medtronic reports full year and fourth quarter fiscal year 2022 financial results 236
6.74 May 26, 2022: Medtronic reports full year and fourth quarter fiscal year 2022 financial results announces 8% dividend increase 237
6.75 May 25, 2022: Baxter Highlights Business Strategies and Innovation at 2022 Investor Conference 237
6.76 May 23, 2022: Edwards Lifesciences to Present at the Bernstein 38th Annual Strategic Decisions Conference 237
6.77 May 12, 2022: Johnson & Johnson to Participate in Goldman Sachs 43rd Annual Global Healthcare Conference 237
6.78 May 12, 2022: Neovasc Announces First Quarter Financial Results and Provides Corporate Update 238
6.79 May 11, 2022: Johnson & Johnson Appoints Thibaut Mongon as CEO Designate of Planned New Consumer Health Company 239
6.80 May 10, 2022: Johnson & Johnson MedTech Adds New CFO to the Mix 240
6.81 May 10, 2022: Baxter to Host 2022 Investor Conference 240
6.82 May 04, 2022: Inari Medical Reports First Quarter 2022 Financial Results 240
6.83 May 02, 2022: Johnson & Johnson to Participate in the Bernstein 38th Annual Strategic Decisions Conference 241
6.84 May 02, 2022: Teleflex to Host Analyst & Investor Day on May 20, 2022 241
6.85 Apr 28, 2022: Boston Scientific reports net sales of $3.03bn in Q1 2022 242
6.86 Apr 28, 2022: Teleflex Reports First Quarter Financial Results And Full Year Outlook 243
6.87 Apr 28, 2022: Abiomed Announces Fourth Quarter Record Revenue of $270 Million, up 12% Year Over Year 244
6.88 Apr 28, 2022: LeMaitre Q1 2022 Financial Results 246
6.89 Apr 27, 2022: Merit Medical Reports Results for Quarter Ended March 31, 2022 247
6.90 Apr 27, 2022: Boston Scientific Announces Results for First Quarter 2022 248
6.91 Apr 26, 2022: Utah Medical Products Reports Financial Performance for First Quarter 2022 251
6.92 Apr 26, 2022: Resolutions at Getinge’s Annual General Meeting 26 April 2022 254
6.93 Apr 21, 2022: Merit Medical Announces Chief Operating Officer Transition 255
6.94 Apr 20, 2022: Johnson & Johnson to Participate in the UBS Global Healthcare Conference 255
6.95 Apr 20, 2022: Inari Medical to Announce First Quarter 2022 Financial Results 256
6.96 Apr 19, 2022: Johnson & Johnson Reports Q1 2022 Results 256
6.97 Apr 19, 2022: Edwards Lifesciences to Host Earnings Conference Call on April 26, 2022 257
6.98 Apr 15, 2022: LeMaitre Will Announce First Quarter 2022 Earnings Results April 28, 2022 257
6.99 Apr 08, 2022: Abiomed Fourth Quarter Fiscal 2022 Earnings and Conference Call Notification 257
6.100 Apr 08, 2022: Getinge announces its Q1 Report 2022 on April 26 at 08:00 CEST followed by a conference call at 10:00 CEST 258
6.101 Apr 07, 2022: Johnson & Johnson to Participate in the BofA Securities 2022 Healthcare Conference 258
6.102 Apr 01, 2022: Japan Lifeline Announces a New Appointment of Operating Officer 258
6.103 Mar 30, 2022: InnovHeart appoints David Wilson as Chief Executive Officer 259
6.104 Mar 21, 2022: Abiomed to Host Investor Call on Impella ECP, the World’s Smallest Heart Pump at 9 French 259
6.105 Mar 15, 2022: Johnson & Johnson to Host Investor Conference Call on First-Quarter Results 260
6.106 Mar 10, 2022: Neovasc Announces Fourth Quarter and Fiscal Year 2021 Financial Results and Provides Corporate Update 260
6.107 Mar 08, 2022: GE Elects Three New Members To Board of Directors 262
6.108 Mar 01, 2022: Inari Medical Appoints Robert Warner to Board of Directors 263
6.109 Feb 25, 2022: Getinge introduces a flexible workplace deal for employees in the UK and Ireland 263
6.110 Feb 24, 2022: Teleflex Announces Fourth Quarter and Full-Year 2021 Financial Results 263
6.111 Feb 24, 2022: LeMaitre Q4 2021 Financial Results 265
6.112 Feb 23, 2022: Inari Medical Reports Fourth Quarter and Full Year 2021 Financial Results 266
6.113 Feb 18, 2022: Shanghai MicroPort Medical Group announces Chairman of the Board of Directors, Chairman of the Supervisory Committee, and Senior Management Announcement 268
6.114 Feb 15, 2022: Johnson & Johnson Names Darius Adamczyk, Chairman and Chief Executive Officer of Honeywell, to its Board of Directors 268
6.115 Feb 14, 2022: Asahi Intecc Co. Announces Differences between Consolidated Earnings Forecasts and Actual Results for the Cumulative Period for the Second Quarter of Fiscal Year Ending June 2022 268
6.116 Feb 11, 2022: LeMaitre Will Announce Fourth Quarter 2021 Earnings Results February 24, 2022 269
6.117 Feb 10, 2022: Inari Medical to Announce Fourth Quarter and Full-Year 2021 Financial Results 269
6.118 Feb 10, 2022: Baxter to Present at Citi’s 2022 Virtual Healthcare Conference 269
6.119 Feb 09, 2022: Johnson & Johnson to Participate in the Raymond James 43rd Annual Institutional Investors Conference 269
6.120 Feb 08, 2022: JMS Announces Financial Results for the 3rd Quarter of FYE March 2022 270
6.121 Feb 08, 2022: Abiomed to Present at the SVB Leerink 11th Annual Global Healthcare Conference 271
6.122 Feb 07, 2022: Johnson & Johnson to Participate in the Cowen 42nd Annual Health Care Virtual Conference 272
6.123 Feb 01, 2022: UTMD Reports Audited Year 2021 and Fourth Quarter Financial Performance 272
6.124 Jan 28, 2022: Japan Lifeline Co. Announces Consolidated Financial Results for the Nine months Ended December 31, 2021 277
6.125 Jan 28, 2022: Westmead Private Hospital unveils next stage in multi-million dollar upgrade 278
7 Appendix 280
7.1 Methodology 280
7.2 About GlobalData 283
7.3 Contact Us 283
7.4 Disclaimer 283
![]()