Coronary Stents – Pipeline Products by Stage of Development 21
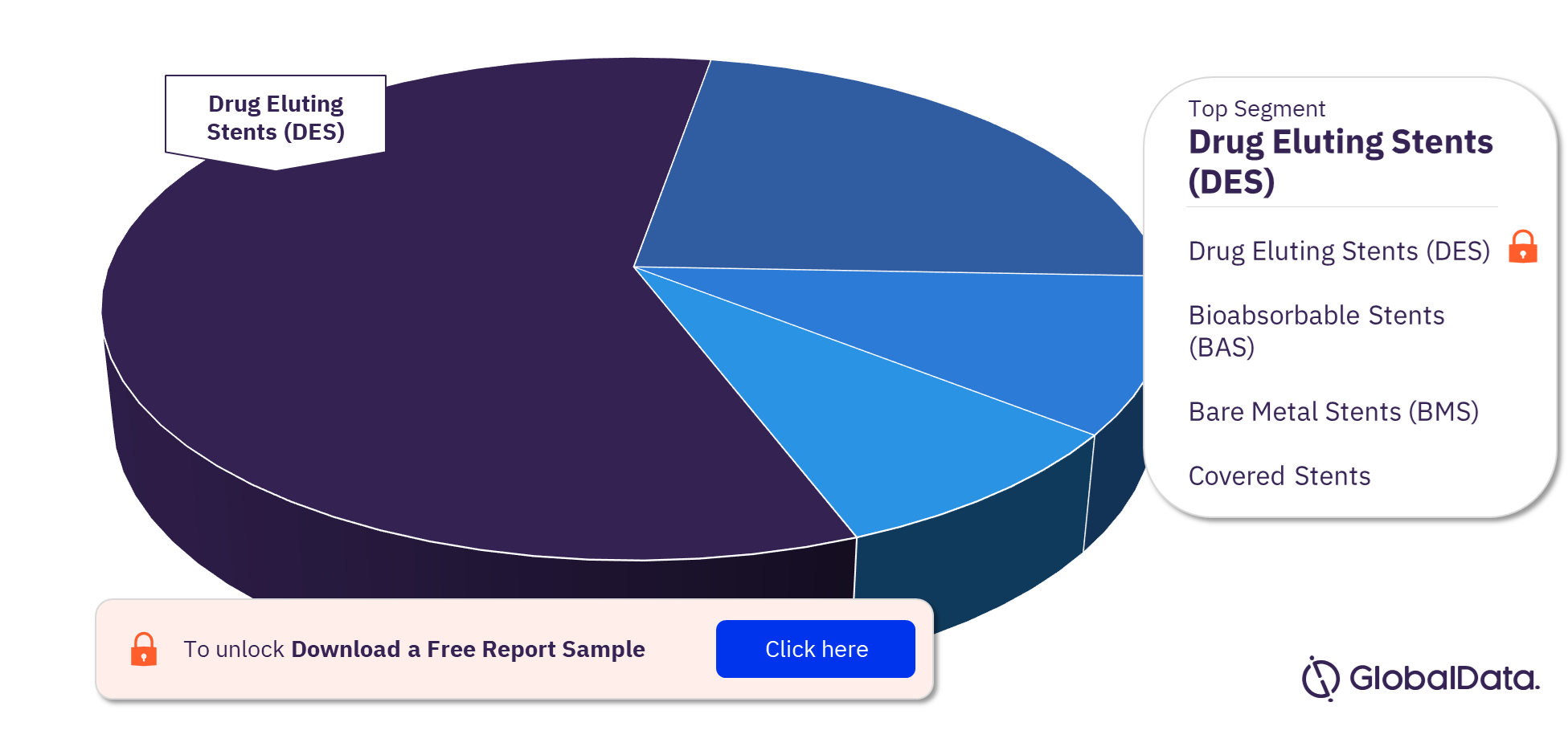
Coronary Stents – Pipeline Products by Segment 22
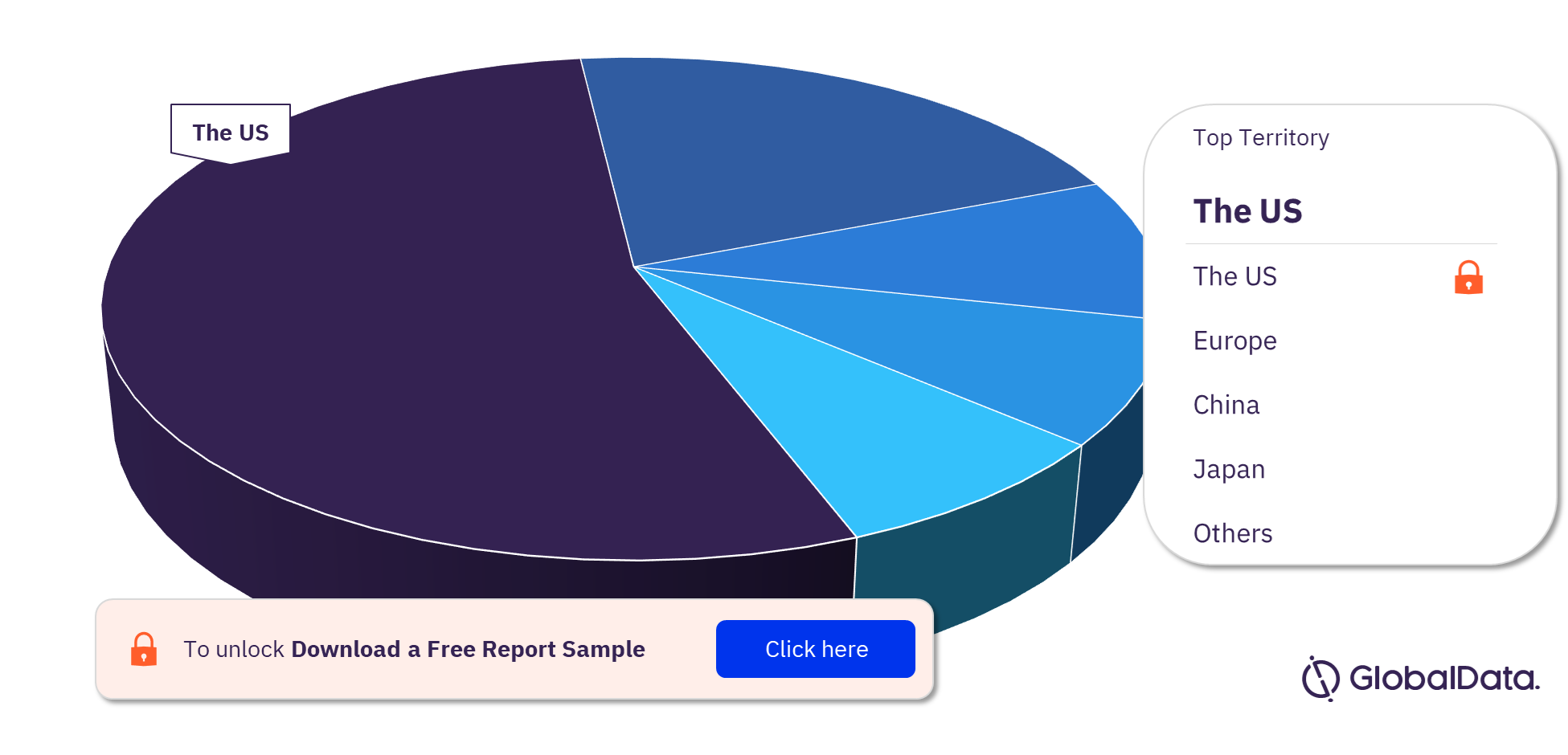
Coronary Stents – Pipeline Products by Territory 23
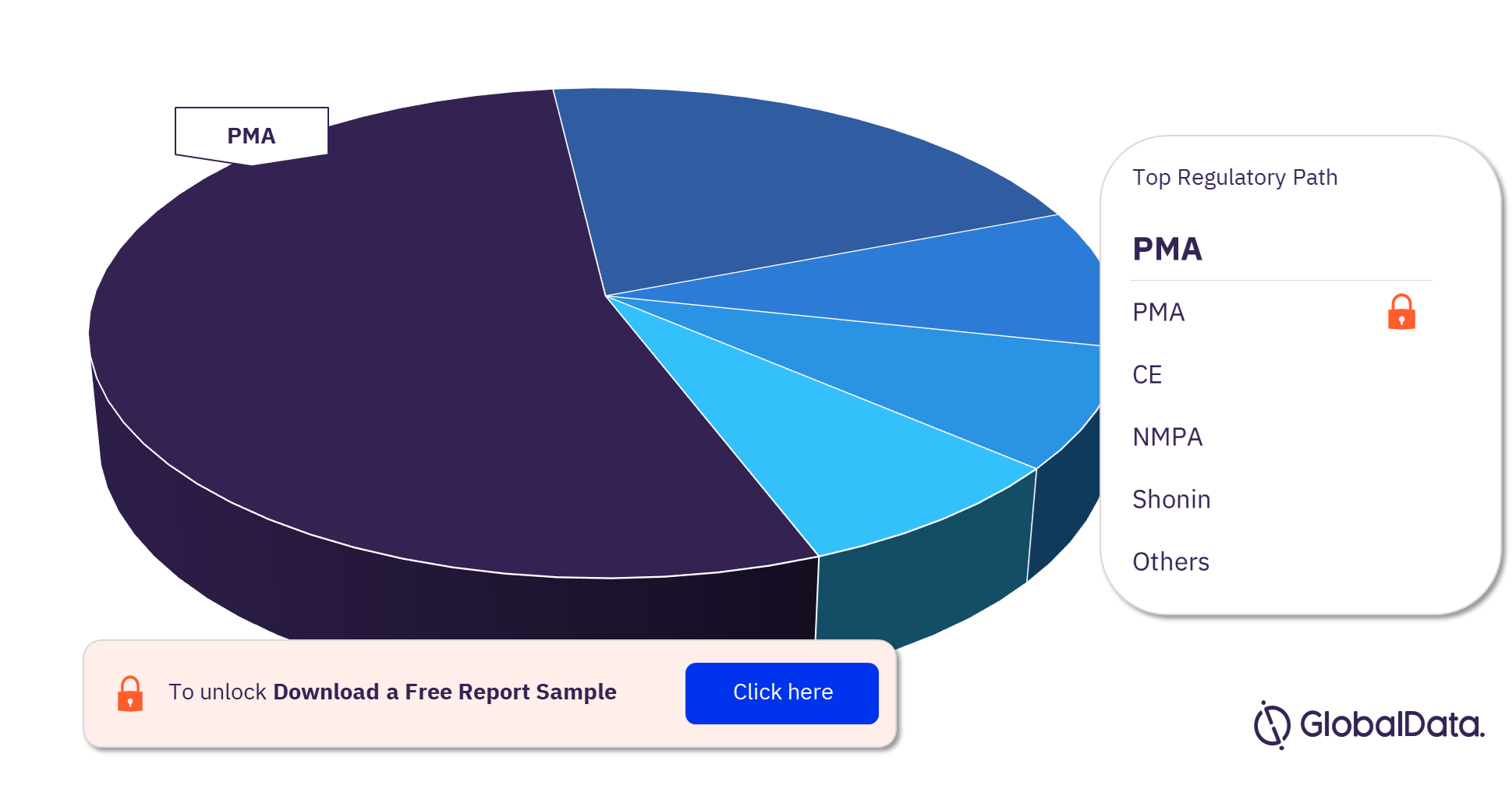
Coronary Stents – Pipeline Products by Regulatory Path 25
Coronary Stents – Pipeline Products by Estimated Approval Date 26
Coronary Stents – Ongoing Clinical Trials 27
Coronary Stents Companies – Pipeline Products by Stage of Development 28
Coronary Stents – Pipeline Products by Stage of Development 32
Abbott Vascular Inc Pipeline Products & Ongoing Clinical Trials Overview 36
Second Generation Bioresorbable Vascular Scaffold – Product Status 36
Second Generation Bioresorbable Vascular Scaffold – Product Description 36
XIENCE BIOPRIME – Product Status 37
XIENCE BIOPRIME – Product Description 37
Xience Max – Product Status 37
Xience Max – Product Description 38
Xience SBA Everolimus Eluting Coronary Stent System – Product Status 38
Xience SBA Everolimus Eluting Coronary Stent System – Product Description 38
XIENCE Thinman DES – Product Status 39
XIENCE Thinman DES – Product Description 39
ZoMaxx Drug Eluting Coronary Stent System – Product Status 39
ZoMaxx Drug Eluting Coronary Stent System – Product Description 40
Abbott Vascular Inc – Ongoing Clinical Trials Overview 41
Xience SBA Everolimus Eluting Coronary Stent System – A Multi-center, Randomized, Controlled Trial to Demonstrate the Safety and Effectiveness of the MiStent II for the Revascularization of Coronary Arteries: CRYSTAL Study 42
Xience SBA Everolimus Eluting Coronary Stent System – A Prospective, Multi-center, Single-blinded, Randomized Trial of the Sirolimus-eluting Iron Bioresorbable Coronary Scaffold System in Patients with Coronary Artery Disease: IRONMAN-II 42
Xience SBA Everolimus Eluting Coronary Stent System – Intracoronary Stenting and Restenosis – Randomized Trial of Drug-eluting Stent Implantation or Drug-coated Balloon Angioplasty According to Neointima Morphology in Drug-eluting Stent Restenosis 5 42
Adcomp Technologies Inc. Pipeline Products & Ongoing Clinical Trials Overview 43
Dual Drug Eluting Stent – Product Status 43
Dual Drug Eluting Stent – Product Description 43
Advanced Bifurcation Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 44
ABS Stenting System – Drug Eluting Stent – Product Status 44
ABS Stenting System – Drug Eluting Stent – Product Description 44
Stenting System – Bare Metal Stent – Product Status 45
Stenting System – Bare Metal Stent – Product Description 45
Aeon Bioscience Pipeline Products & Ongoing Clinical Trials Overview 46
Drug Eluting Stent – Product Status 46
Drug Eluting Stent – Product Description 46
Amaranth Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 47
80-micron MAGNITUDE Bioresorbable Scaffold – Product Status 47
80-micron MAGNITUDE Bioresorbable Scaffold – Product Description 47
APTITUDE Sirolimus-Eluting Bioresorbable Scaffold – Product Status 48
APTITUDE Sirolimus-Eluting Bioresorbable Scaffold – Product Description 48
DEFIANCE Scaffold – Product Status 48
DEFIANCE Scaffold – Product Description 49
FORTITUDE Scaffold – Product Status 49
FORTITUDE Scaffold – Product Description 49
MAGNITUDE Sirolimus-Eluting Bioresorbable Scaffold – Product Status 50
MAGNITUDE Sirolimus-Eluting Bioresorbable Scaffold – Product Description 50
Second Generation FORTITUDE Scaffold – Product Status 50
Second Generation FORTITUDE Scaffold – Product Description 51
Amaranth Medical Inc – Ongoing Clinical Trials Overview 52
APTITUDE Sirolimus-Eluting Bioresorbable Scaffold – Restoring Endoluminal Narrowing Using Bioresorbable Scaffolds – Extended Trial II 53
MAGNITUDE Sirolimus-Eluting Bioresorbable Scaffold – Restoring Endoluminal Narrowing Using Bioresorbable Scaffolds – Extended Trial III 54
Arterius Ltd Pipeline Products & Ongoing Clinical Trials Overview 55
Arteriosorb Absorbable Drug-Eluting Scaffold – Product Status 55
Arteriosorb Absorbable Drug-Eluting Scaffold – Product Description 55
Atrium Medical Corp Pipeline Products & Ongoing Clinical Trials Overview 56
Next Generation Super Flexible Cobalt Chromium Coronary Stent – Product Status 56
Next Generation Super Flexible Cobalt Chromium Coronary Stent – Product Description 56
Axordia Ltd (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 57
Regenerative Stent – Product Status 57
Regenerative Stent – Product Description 57
B. Braun Melsungen AG Pipeline Products & Ongoing Clinical Trials Overview 58
Coroflex DEBlue – Product Status 58
Coroflex DEBlue – Product Description 58
Bactiguard Holding AB Pipeline Products & Ongoing Clinical Trials Overview 59
Metal-Coated Vascular Stent – Product Status 59
Metal-Coated Vascular Stent – Product Description 59
Bionext Biotech Products Ltd. Pipeline Products & Ongoing Clinical Trials Overview 60
Biostent – Product Status 60
Biostent – Product Description 60
Biosensors International Group Ltd Pipeline Products & Ongoing Clinical Trials Overview 61
BioFreedom Ultra – Product Status 61
BioFreedom Ultra – Product Description 61
Sparrow Drug Eluting Stent System – Product Status 62
Sparrow Drug Eluting Stent System – Product Description 62
Biosensors International Group Ltd – Ongoing Clinical Trials Overview 63
BioFreedom Ultra – A Pilot Study Registry of the BioFreedom BA9 Ultra Drug-Coated Coronary Stent for Patients With ST Elevation Myocardial Infarct (STEMI) Undergoing Percutaneous Coronary Intervention (PCI) 64
BioFreedom Ultra – A Post-Market Registry of the BioFreedom Ultra CoCr Biolimus A9 Coated Coronary Stent System 64
BioFreedom Ultra – A Prospective Multicenter Single Arm Trial to Assess the Safety and Effectiveness of Additional Sizes of the BioFreedom Ultra CoCr Biolimus A9 Coated Coronary Stent System 64
BioFreedom Ultra – A Prospective Study of the BioFreedom Biolimus A9 Drug Coated Stent in Patients at High Risk for Bleeding 65
BioFreedom Ultra – A Randomized Controlled Comparison Between One Versus More than Six Months of Dual Antiplatelet Therapy after Biolimus A9-eluting Stent Implantation 65
BioFreedom Ultra – Asian Registry of the BioFreedom BA9 Drug-Coated Coronary Stent for Patients with ST Elevation Myocardial Infarction (STEMI) Undergoing Percutaneous Coronary Intervention (PCI) 65
BioFreedom Ultra – BioFreedom Ultra Stent in Hong Kong All Comers Registry 66
BioFreedom Ultra – Comparison of Polymer-free Cobalt-Chromium Thin Drug-coated Stents with Biodegradable Polymer Ultrathin Sirolimus-Eluting Stents and Prasugrel Monotherapy with Conventional 12-Month Dual Antiplatelet Therapy 66
BioFreedom Ultra – Evaluation of Effectiveness and Safety of Biofreedom Family Stent in Routine Clinical Practice; A Multicenter, Prospective Observational Study 66
BioFreedom Ultra – Evaluation of Effectiveness and Safety of the First, Second, and New Drug-eluting Stents in Routine Clinical Practice 67
BioFreedom Ultra – P2Y12 Inhibitor-based Single Antiplatelet Therapy Versus Conventional Dual Antiplatelet Therapy After Percutaneous Coronary Intervention With BioFreedom Ultra Drug-coated Stent for Unprotected Left Main Coronary Artery Disease (ULTRA-LM) 67
BioFreedom Ultra – Randomized Comparison of a Polymer-free Biolimus-eluting BioFreedom Stent with a Biodegradable-polymer Sirolimus-eluting Orsiro Stent in Patients Treated with Percutaneous Coronary Intervention 67
BioFreedom Ultra – Randomized Comparison of Vascular Healing of a Polymer-free Biolimus-eluting BIOFREEDOM Stent with a Biodegradable-polymer Sirolimus-eluting ORSIRO Stent in Patients with ST-segment Elevation Myocardial Infarction 68
Biosten, LLC Pipeline Products & Ongoing Clinical Trials Overview 69
Biodegradable Endovascular Implant – Product Status 69
Biodegradable Endovascular Implant – Product Description 69
Biotronik AG Pipeline Products & Ongoing Clinical Trials Overview 70
Coronary Stent System – Product Status 70
Coronary Stent System – Product Description 70
DREAMS 3G System – Product Status 71
DREAMS 3G System – Product Description 71
Magmaris Bioresorbable Magnesium Scaffold – Product Status 71
Magmaris Bioresorbable Magnesium Scaffold – Product Description 72
Biotronik AG – Ongoing Clinical Trials Overview 73
Magmaris Bioresorbable Magnesium Scaffold – A Sirolimus Eluting Bioresorbable Magnesium Stent for Treatment of Coronary Bifurcation Lesions: The BIFSORB Pilot Study II 74
Magmaris Bioresorbable Magnesium Scaffold – BIOTRONIK – Safety and Clinical Performance of the Drug Eluting Absorbable Metal Scaffold (DREAMS 2nd Generation) in the Treatment of Subjects with de Novo Lesions in Native Coronary Arteries: BIOSOLVE-II 74
Magmaris Bioresorbable Magnesium Scaffold – Biotroniks – Safety and Performance in de Novo Lesion of Native Coronary Arteries with Magmaris- Registry: BIOSOLVE-IV 74
Magmaris Bioresorbable Magnesium Scaffold – Coronary Artery Healing Process after Bioresorbable Scaffold in Patients with Non-ST-segment Elevation Myocardial Infarction (NSTEMI) 75
Magmaris Bioresorbable Magnesium Scaffold – Optical Coherence Guided Treatment of ST-segment Elevation Myocardial Infarction with the Drug-eluting Resorbable Magnesium Scaffold: The BEST- MAG Multicentre Study (Belgian ST-segment Elevation Myocardial Infarction Treatment with Resorbable Magnesium Scaffold) 75
Magmaris Bioresorbable Magnesium Scaffold – Retrospective, Observational Register to Investigate the Procedural and Post Procedural Implantation of Bioabsorbable Magnesium Scaffolds Magmaris (MAGIC Registry) 75
Magmaris Bioresorbable Magnesium Scaffold – RMS (Resorbable Magnesium Scaffolds) Registry 76
Magmaris Bioresorbable Magnesium Scaffold – Scaffold Implantation in Emilia-Romagna 76
DREAMS 3G System – BIOTRONIK – Safety and Clinical Performance of the – Sirolimus-Eluting Resorbable Coronary Magnesium Scaffold System (DREAMS 3G) in the Treatment of Subjects with de Novo Lesions in Native Coronary Arteries 77
DREAMS 3G System – BIOTRONIK – Safety and Clinical Performance of the Drug Eluting Resorbable Coronary Magnesium Scaffold System (DREAMS 3G) in the Treatment of Subjects with de Novo Lesions in Native Coronary Arteries: BIOMAG-II: A Randomized Controlled Trial 77
Biotronik SE & Co KG Pipeline Products & Ongoing Clinical Trials Overview 78
AMS-2 – Product Status 78
AMS-2 – Product Description 78
ProGenic Pimecrolimus-eluting Coronary Stent System – Product Status 79
ProGenic Pimecrolimus-eluting Coronary Stent System – Product Description 79
Biotyx Medical (Shenzhen) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 80
IBS Angel – Product Status 80
IBS Angel – Product Description 80
Biotyx Medical (Shenzhen) Co Ltd – Ongoing Clinical Trials Overview 81
IBS Angel – A Prospective, Multi-center, Single-arm Clinical Trial to Evaluate the Safety and Efficacy of Iron Bioresorbable Scaffold System (IBS Angel) in Patients with Pulmonary Artery Stenosis 82
Boston Scientific Corp Pipeline Products & Ongoing Clinical Trials Overview 83
Fully Resorbable Drug-Eluting Scaffold System – Product Status 83
Fully Resorbable Drug-Eluting Scaffold System – Product Description 84
JACTAX Drug Eluting Stent – Product Status 84
JACTAX Drug Eluting Stent – Product Description 84
SYNERGY – High Bleeding Risk – Product Status 85
SYNERGY – High Bleeding Risk – Product Description 85
SYNERGY 48 Stent – Product Status 85
SYNERGY 48 Stent – Product Description 86
SYNERGY XD Stent – Product Status 86
SYNERGY XD Stent – Product Description 86
TAXUS Petal Bifurcation Paclitaxel-Eluting Stent System – Product Status 87
TAXUS Petal Bifurcation Paclitaxel-Eluting Stent System – Product Description 87
Boston Scientific Corp – Ongoing Clinical Trials Overview 88
SYNERGY XD Stent – Evaluation of Effectiveness and Safety of Synergy™ XD Stent and Synergy Megatron™ Stent in Routine Clinical Practice; A Multicenter, Prospective Observational Study 89
Cardionovum GmbH Pipeline Products & Ongoing Clinical Trials Overview 90
Cardiosorb – Product Status 90
Cardiosorb – Product Description 90
DEBLIMUS – Product Status 91
DEBLIMUS – Product Description 91
PROTECT – Product Status 91
PROTECT – Product Description 91
ReNATURAL (M) – Product Status 92
ReNATURAL (M) – Product Description 92
ReNATURAL (P) – Product Status 92
ReNATURAL (P) – Product Description 93
Cardiorev Pte Ltd (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 94
Peptide-Eluting Coronary Stent – Product Status 94
Peptide-Eluting Coronary Stent – Product Description 94
Columbia University Pipeline Products & Ongoing Clinical Trials Overview 95
C3 Exoenzyme Coated Stent – Product Status 95
C3 Exoenzyme Coated Stent – Product Description 95
Concept Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 96
Abluminus DES+ – Product Status 96
Abluminus DES+ – Product Description 96
Concept Medical Inc – Ongoing Clinical Trials Overview 97
Abluminus DES+ – A Multicenter, Prospective, Study to Evaluate the Safety and Effcacy of the Abluminus DES+ in an All-Comers Population 98
Abluminus DES+ – A Post Market Registry of Abluminus Sirolimus Eluting Coronary Stent System for Percutaneous Intervention in Patients with Diabetes Mellitus 98
Abluminus DES+ – ABILITY Diabetes Global 98
Abluminus DES+ – ABLUMINUS Below the Knee (BTK) Drug Eluting Stent (DES) Registry (ABLUMINUS BTK) – First in Men 99
Abluminus DES+ – Randomized Trial Investigating Clinical Outcomes of Two Sirolimus-Eluting Stents in Diabetes Mellitus 99
Contego Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 100
CORGUARD Coronary Stent System – Product Status 100
CORGUARD Coronary Stent System – Product Description 100
Cordis Corp Pipeline Products & Ongoing Clinical Trials Overview 101
Corio Pimecrolimus-Eluting Stent – Product Status 101
Corio Pimecrolimus-Eluting Stent – Product Description 101
Cypher Elite – Product Status 102
Cypher Elite – Product Description 102
NEVO Sirolimus-Eluting Coronary Stent – Product Status 102
NEVO Sirolimus-Eluting Coronary Stent – Product Description 103
Next Generation Coronary Stent – Product Status 103
Next Generation Coronary Stent – Product Description 103
SymBio Pimecrolimus/Paclitaxel-Eluting Stent – Product Status 104
SymBio Pimecrolimus/Paclitaxel-Eluting Stent – Product Description 104
Cytograft Tissue Engineering Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 105
LifeJacket Stent Graft – Coronary Stenting – Product Status 105
LifeJacket Stent Graft – Coronary Stenting – Product Description 105
DISA Vascular (Pty) Ltd Pipeline Products & Ongoing Clinical Trials Overview 106
Stellium Stent – Product Status 106
Stellium Stent – Product Description 106
Elixir Medical Corp Pipeline Products & Ongoing Clinical Trials Overview 107
DESolve Myolimus Eluting Bioresorbable Coronary Scaffold System – Product Status 107
DESolve Myolimus Eluting Bioresorbable Coronary Scaffold System – Product Description 107
Myolimus Eluting Coronary Stent – Durable Polymer – Product Status 108
Myolimus Eluting Coronary Stent – Durable Polymer – Product Description 108
Endomimetics LLC Pipeline Products & Ongoing Clinical Trials Overview 109
Coronary Artery Stent – Product Status 109
Coronary Artery Stent – Product Description 109
Envision Scientific Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 110
Paclitaxel Eluting Stent – Product Status 110
Paclitaxel Eluting Stent – Product Description 110
HangZhou HuaAn Biotechnology Co., Ltd. Pipeline Products & Ongoing Clinical Trials Overview 111
Xinsorb BRS – Product Status 111
Xinsorb BRS – Product Description 111
I.B.S. S.p.A. Pipeline Products & Ongoing Clinical Trials Overview 112
Drug Eluting Stent – Product Status 112
Drug Eluting Stent – Product Description 112
Icon Interventional Systems, Inc. Pipeline Products & Ongoing Clinical Trials Overview 113
Biosorb Pediatric Dissolving Stent – Product Status 113
Biosorb Pediatric Dissolving Stent – Product Description 113
ID Nest Medical SAS Pipeline Products & Ongoing Clinical Trials Overview 114
ID Arterial System – Product Status 114
ID Arterial System – Product Description 114
InspireMD Inc Pipeline Products & Ongoing Clinical Trials Overview 115
MGuard Drug Eluting Stent – Product Status 115
MGuard Drug Eluting Stent – Product Description 115
Intressa Vascular SA Pipeline Products & Ongoing Clinical Trials Overview 116
Allay Aortic Stent – Product Status 116
Allay Aortic Stent – Product Description 116
Japan Stent Technology Co., Ltd. Pipeline Products & Ongoing Clinical Trials Overview 117
Bioabsorbable Stent – Product Status 117
Bioabsorbable Stent – Product Description 117
JW Medical Systems Ltd Pipeline Products & Ongoing Clinical Trials Overview 118
Excel II DES – Product Status 118
Excel II DES – Product Description 118
JW Medical Systems Ltd – Ongoing Clinical Trials Overview 119
Excel II DES – A Prospective Multicenter Randomized Trial to Assess the Safety and Effectiveness of EXCEL-II Sirolimus Eluting Stent vs. EXCEL Sirolimus Eluting Stent for the Treatment of Patients with de Novo Coronary Artery Lesions (CREDIT II Trial) 120
Kaneka Corp Pipeline Products & Ongoing Clinical Trials Overview 121
MAHOROBA Stent – Product Status 121
MAHOROBA Stent – Product Description 121
Kyoto Medical Planning Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 122
Igaki-Tamai Coronary Stent – Product Status 122
Igaki-Tamai Coronary Stent – Product Description 122
Lifetech Scientific (Shenzhen) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 123
IBS Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold System – Product Status 123
IBS Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold System – Product Description 123
Lifetech Scientific (Shenzhen) Co Ltd – Ongoing Clinical Trials Overview 124
IBS Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold System – A First-in-man Study of Sirolimus-eluting Iron Bioresorbable Coronary Scaffold System (IBS) 125
IBS Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold System – A Prospective, Multi-center, Single-arm Trial of the Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold System in Patients with Coronary Artery Disease: IRONMAN-III 125
IBS Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold System – A Prospective, Multi-center, Single-blinded, Randomized Trial of the Sirolimus-eluting Iron Bioresorbable Coronary Scaffold System in Patients with Coronary Artery Disease: IRONMAN-II 125
IBS Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold System – A Prospective, Non-randomized, Open-label, Non-comparative, First-in-Man Study to Evaluate the Feasibility and Safety of Sirolimus-eluting Iron Bioresorbable Coronary Scaffold System 126
IBS Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold System – Feasibility, Efficacy and Safety of IBS for Implantation in the PDA in Duct-dependent Cyanotic CHD 126
MangoGen Pharma Inc Pipeline Products & Ongoing Clinical Trials Overview 127
Gene-Delivering Stent – Product Status 127
Gene-Delivering Stent – Product Description 127
Manli Cardiology Ltd Pipeline Products & Ongoing Clinical Trials Overview 128
Mirage – Product Status 128
Mirage – Product Description 128
Medinol Ltd Pipeline Products & Ongoing Clinical Trials Overview 129
Elunir Elastomer Drug Eluting Stent – 38mm – Product Status 129
Elunir Elastomer Drug Eluting Stent – 38mm – Product Description 130
Elunir Elastomer Drug Eluting Stent – 44mm – Product Status 130
Elunir Elastomer Drug Eluting Stent – 44mm – Product Description 131
IoNIR Ridaforolimus-Eluting Coronary Stent System – Product Status 131
IoNIR Ridaforolimus-Eluting Coronary Stent System – Product Description 131
Medinol Ltd – Ongoing Clinical Trials Overview 132
IoNIR Ridaforolimus-Eluting Coronary Stent System – IonMAN Trial-First In Human Study of the IoNIR Ridaforolimus-Eluting Coronary Stent System 133
Medlogics Device Corp (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 134
COBRA-Q Drug Eluting Stent – Product Status 134
COBRA-Q Drug Eluting Stent – Product Description 134
SYNERGY – Product Status 135
SYNERGY – Product Description 135
Medtronic Plc Pipeline Products & Ongoing Clinical Trials Overview 136
Drug Filled Stent – Product Status 136
Drug Filled Stent – Product Description 137
Resolute Onyx DES – DAPT – Product Status 137
Resolute Onyx DES – DAPT – Product Description 137
Medtronic Plc – Ongoing Clinical Trials Overview 138
Resolute Onyx DES – DAPT – BIOTRONIK- A Prospective, Randomised, Multi-center Study to Assess the Safety of the Orsiro Mission Stent Compared to Resolute Onyx Stent in Subjects at High Risk of Bleeding in Combination With 1-month Dual Antiplatelet Therapy (DAPT) 139
Meril Life Sciences Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 140
MeRes100 – Product Status 140
MeRes100 – Product Description 140
Meriskin BRS – Product Status 141
Meriskin BRS – Product Description 141
Meril Life Sciences Pvt Ltd – Ongoing Clinical Trials Overview 142
MeRes100 – A Multi Center Randomized Control Study of MeRes100 Sirolimus Eluting BioResorbable Vascular Scaffold System in Treatment of Coronary Artery Disease Patients: MeRes – China 143
MeRes100 – A Prospective, Multinational, Multicenter, Single Arm, Open Label, Pilot Clinical Study of MeRes100 Sirolimus Eluting Bioresorbable Vascular Scaffold System in the Treatment of De-novo Native Coronary Artery Lesions: MeRes-1 Extend 143
MeRes100 – To Compare the Safety and Performance of MeRes100 Sirolimus Eluting BioResorbable Vascular Scaffold System Versus Contemporary DES Platforms in Patients With de Novo Coronary Artery Lesions 143
Miami Cardiovascular Innovations Pipeline Products & Ongoing Clinical Trials Overview 144
Biologically Engineered Stent – Product Status 144
Biologically Engineered Stent – Product Description 144
Micell Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 145
Bioresorbable Scaffold (BRS) – Product Status 145
Bioresorbable Scaffold (BRS) – Product Description 145
Michigan Technological University Pipeline Products & Ongoing Clinical Trials Overview 146
Bioabsorbable Stent – Product Status 146
Bioabsorbable Stent – Product Description 146
MicroPort Scientific Corp Pipeline Products & Ongoing Clinical Trials Overview 147
Fantasy Drug-Eluting Stent – Product Status 147
Fantasy Drug-Eluting Stent – Product Description 147
Firebird Pro+ – Product Status 148
Firebird Pro+ – Product Description 148
Firebird2 Pro – Product Status 148
Firebird2 Pro – Product Description 148
Firefalcon Bioresorbable Device – Product Status 149
Firefalcon Bioresorbable Device – Product Description 149
Firehawk Plus – Product Status 149
Firehawk Plus – Product Description 149
Firesorb Bioresorbable Scaffold – Product Status 150
Firesorb Bioresorbable Scaffold – Product Description 150
MicroPort Scientific Corp – Ongoing Clinical Trials Overview 151
Firesorb Bioresorbable Scaffold – A First-in-man Study of the Firesorb Sirolimus Target Eluting Bioresorbable Vascular Scaffold in Patients with Coronary Artery Disease: FUTURE-I 152
Firesorb Bioresorbable Scaffold – A Prospective Multicenter Single-arm Clinical Trial Assessing the Safety and Effectiveness of Firesorb Sirolimus Target Eluting Bioresorbable Vascular Scaffold for the Treatment of Coronary Artery Disease: FUTURE III 152
Firesorb Bioresorbable Scaffold – A Randomized Trial of the Firesorb Sirolimus Target Eluting Bioresorbable Vascular Scaffold in Patients with Coronary Artery Disease: FUTURE-II 152
Minvasys SAS Pipeline Products & Ongoing Clinical Trials Overview 153
Nile LM SIR Sirolimus Eluting Intracoronary Stent – Product Status 153
Nile LM SIR Sirolimus Eluting Intracoronary Stent – Product Description 153
MIV Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 154
Smart-1 DES – Product Status 154
Smart-1 DES – Product Description 154
Smart-2 DES – Product Status 155
Smart-2 DES – Product Description 155
Smart-3 DES – Product Status 155
Smart-3 DES – Product Description 156
Smart-4 DES – Product Status 156
Smart-4 DES – Product Description 156
Vestapor – Product Status 157
Vestapor – Product Description 157
VESTAsync – Product Status 157
VESTAsync – Product Description 158
NanoCoeur, Inc. Pipeline Products & Ongoing Clinical Trials Overview 159
Nanocoating Cardiac Stent – Product Status 159
Nanocoating Cardiac Stent – Product Description 159
Nanova, Inc Pipeline Products & Ongoing Clinical Trials Overview 160
Nanova Stent – Product Status 160
Nanova Stent – Product Description 160
Neovasc Inc Pipeline Products & Ongoing Clinical Trials Overview 161
Coronary Bifurcation Stent Delivery System – Product Status 161
Coronary Bifurcation Stent Delivery System – Product Description 161
Nesstent Ltd. Pipeline Products & Ongoing Clinical Trials Overview 162
NesStent’s Stent – Coronary Bifurcations – Product Status 162
NesStent’s Stent – Coronary Bifurcations – Product Description 162
Nexeon MedSystems Inc Pipeline Products & Ongoing Clinical Trials Overview 163
RIVIERA Coronary Stent System – Product Status 163
RIVIERA Coronary Stent System – Product Description 163
North Carolina State University Pipeline Products & Ongoing Clinical Trials Overview 164
Biodegradable Metal Stent – Product Status 164
Biodegradable Metal Stent – Product Description 164
Northwestern University Pipeline Products & Ongoing Clinical Trials Overview 165
Liquid Cast Drug-Eluting Biodegradable Stent – Product Status 165
Liquid Cast Drug-Eluting Biodegradable Stent – Product Description 165
NuVascular Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 166
NuSpun DE Stent Sheath – Product Status 166
NuSpun DE Stent Sheath – Product Description 166
OrbusNeich Pipeline Products & Ongoing Clinical Trials Overview 167
Combo Plus CoCr – Product Status 167
Combo Plus CoCr – Product Description 167
Cura Stent – Product Status 168
Cura Stent – Product Description 168
Sirolimus-Eluting Absorbable Vascular Scaffold – Product Status 168
Sirolimus-Eluting Absorbable Vascular Scaffold – Product Description 169
Palmaz Scientific Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 170
Micro-Grooved Coronary Stent – Product Status 170
Micro-Grooved Coronary Stent – Product Description 170
Pediastent LLC Pipeline Products & Ongoing Clinical Trials Overview 171
Pediatric Bioresorbable Stent – Product Status 171
Pediatric Bioresorbable Stent – Product Description 171
QualiMed Innovative Medizinprodukte GmbH Pipeline Products & Ongoing Clinical Trials Overview 172
Biodegradable Coronary Stent – Product Status 172
Biodegradable Coronary Stent – Product Description 172
Qvanteq AG Pipeline Products & Ongoing Clinical Trials Overview 173
Qstent Coronary Stent System – Product Status 173
Qstent Coronary Stent System – Product Description 173
Relisys Medical Devices Ltd Pipeline Products & Ongoing Clinical Trials Overview 174
Corel + C Drug Eluting Stent – Product Status 174
Corel + C Drug Eluting Stent – Product Description 174
REVA Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 175
Fantom Sirolimus-Eluting Bioresorbable Coronary Scaffold – Product Status 175
Fantom Sirolimus-Eluting Bioresorbable Coronary Scaffold – Product Description 176
ReZolve Sirolimus-Eluting Bioresorbable Coronary Scaffold – Product Status 176
ReZolve Sirolimus-Eluting Bioresorbable Coronary Scaffold – Product Description 176
ReZolve2 Drug Eluting Bioresorbable Coronary Scaffold – Product Status 177
ReZolve2 Drug Eluting Bioresorbable Coronary Scaffold – Product Description 177
REVA Medical Inc – Ongoing Clinical Trials Overview 178
Fantom Sirolimus-Eluting Bioresorbable Coronary Scaffold – Expanded FANTOM II Clinical Study 179
Fantom Sirolimus-Eluting Bioresorbable Coronary Scaffold – Post Market Study of the FANTOM Sirolimus-eluting Bioresorbable Coronary Scaffold 179
Fantom Sirolimus-Eluting Bioresorbable Coronary Scaffold – Safety and Efficacy of the FANTOM ENCORE Sirolimus-eluting Bioresorbable Scaffold for Treatment of De-novo Coronary Artery Disease: The ENCORE-I Study 179
Fantom Sirolimus-Eluting Bioresorbable Coronary Scaffold – Safety and Performance Study of the FANTOM Sirolimus-eluting Bioresorbable Coronary Scaffold 180
Fantom Sirolimus-Eluting Bioresorbable Coronary Scaffold – Vascular Healing Pattern, Vasoreactivity, and Quality of Life in Patients with ST Segment Elevation Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention with Sirolimus Eluting FANTOM Bioresorbable Vascular Scaffold with Long Term Clinical, Near Infrared Spectroscopy and Optical Coherence Tomography Follow-up: A FANTOM STEMI Pilot Study 180
Rontis AG Pipeline Products & Ongoing Clinical Trials Overview 181
Bifurcation Stent System – Product Status 181
Bifurcation Stent System – Product Description 181
S3V Vascular Technologies Pipeline Products & Ongoing Clinical Trials Overview 182
3V Avatar – Product Status 182
3V Avatar – Product Description 182
Sahajanand Medical Technologies Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 183
Next-Generation Stent – Product Status 183
Next-Generation Stent – Product Description 183
Shandong Rientech Medical Tech Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 184
Absorbable Zinc Alloy Drug Eluting Coronary Stent System – Product Status 184
Absorbable Zinc Alloy Drug Eluting Coronary Stent System – Product Description 184
Shandong Rientech Medical Tech Co Ltd – Ongoing Clinical Trials Overview 185
Absorbable Zinc Alloy Drug Eluting Coronary Stent System – Exploratory Study on the Safety and Effectiveness of Absorbable Zinc Alloy Drug-eluting Coronary Stent System 186
Shanghai Bio-heart Biological Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 187
Bioheart BRS System – Product Status 187
Bioheart BRS System – Product Description 187
Bioheart Ultra BRS System – Product Status 188
Bioheart Ultra BRS System – Product Description 188
Shanghai Bio-heart Biological Technology Co Ltd – Ongoing Clinical Trials Overview 189
Bioheart BRS System – A Randomized Controlled Trial of the Bioheart Rapamycin Drug-Eluting Bioresorbable Coronary Stent System in Patients With Coronary Artery Disease: BIOHEART-II 190
Bioheart BRS System – A Registry Trial of the Bioheart Rapamycin Drug-eluting Bioresorbable Coronary Stent System in Patients with Coronary Artery Disease: BIOHEART III 190
Bioheart BRS System – Study of Bioheart BRS System for the Treatment of Coronary Artery Disease 190
Shanghai BIOMAGIC Medical Device Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 191
BIOMAGIC Biroresorbable Coronary Stent – Product Status 191
BIOMAGIC Biroresorbable Coronary Stent – Product Description 191
Shanghai Healing Medical Instruments Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 192
Biodegradable Sinus Stent – Product Status 192
Biodegradable Sinus Stent – Product Description 192
Shanghai MicroPort Medical Group Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 193
Firehawk Rapamycin Target Eluting Coronary Stent System – Product Status 193
Firehawk Rapamycin Target Eluting Coronary Stent System – Product Description 193
Shanghai MicroPort Medical Group Co Ltd – Ongoing Clinical Trials Overview 194
Firehawk Rapamycin Target Eluting Coronary Stent System – A Prospective Multi-center Open-label Controlled Trial of Comparison 3 Versus 12 Months of Dual Anti-platelet Therapy after Implantation of Firehawk Sirolimus Target- eluting Stent in Patients with Stable Coronary Artery Disease 195
Firehawk Rapamycin Target Eluting Coronary Stent System – A Prospective Multicenter Post Market Trial to Assess the Safety and Effectiveness of the Firehawk Rapamycin Target Eluting Cobalt Chromium Coronary Stent System (Firehawk Stent System) for the Treatment of Atherosclerotic Lesion(s) 195
Firehawk Rapamycin Target Eluting Coronary Stent System – A Prospective, Multicenter, Single-arm Trail in Evaluating the Safety and Efficacy of the Rapamycin Target Eluting Stent in Patients with Coronary Artery Stenosis 195
Firehawk Rapamycin Target Eluting Coronary Stent System – A Prospective, Open Label, Multi-center Trial of Firehawk Coronary Stent System in the Treatment of Coronary Chronic Total Artery Occlusion Lesion(S) by Optical Coherent Tomography (OCT) and Coronary Angiography 196
Firehawk Rapamycin Target Eluting Coronary Stent System – Assessment of In-stent Intimal Repair and Vessel Reaction After Firehawk Sirolimus Eluting Stent Implantation of STEMI Subjects – An Optical Coherence Tomography (OCT) Study 196
Firehawk Rapamycin Target Eluting Coronary Stent System – Evaluation of Effectiveness and Safety of the First, Second, and New Drug-eluting Stents in Routine Clinical Practice 196
![]()