Diagnostic and Measurement Devices – Pipeline Products by Stage of Development 25
Diagnostic and Measurement Devices – Pipeline Products by Segment 26
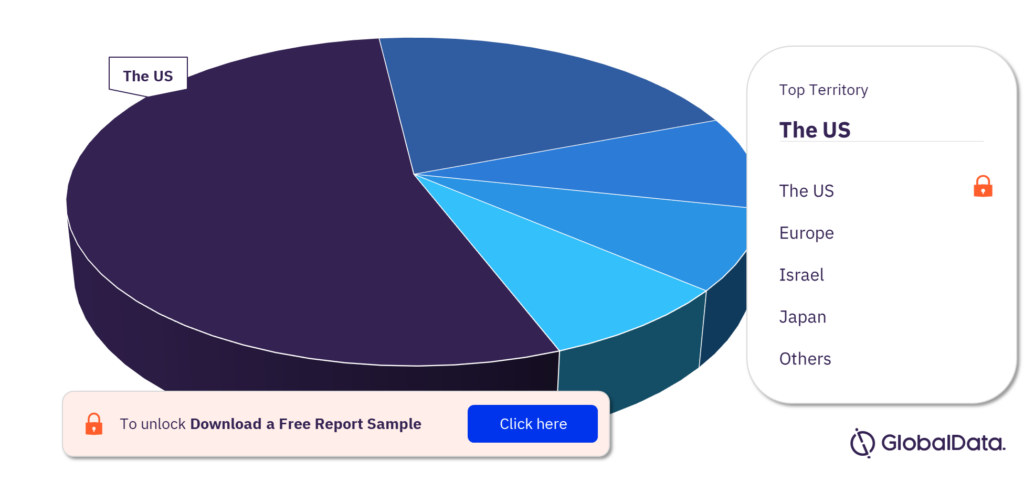
Diagnostic and Measurement Devices – Pipeline Products by Territory 28
Diagnostic and Measurement Devices – Pipeline Products by Regulatory Path 29
Diagnostic and Measurement Devices – Pipeline Products by Estimated Approval Date 30
Diagnostic and Measurement Devices – Ongoing Clinical Trials 31
Diagnostic and Measurement Devices Companies – Pipeline Products by Stage of Development 32
Diagnostic and Measurement Devices – Pipeline Products by Stage of Development 38
Advanced Brain Monitoring Inc Pipeline Products & Ongoing Clinical Trials Overview 43
Apnea Risk Evaluation System – Pediatrics – Product Status 43
Apnea Risk Evaluation System – Pediatrics – Product Description 43
Mandibular Repositioning Device – Product Status 44
Mandibular Repositioning Device – Product Description 44
Advanced Circulatory Systems Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 45
CirQlator Intrathoracic Pressure Regulator – Pediatric Version – Product Status 45
CirQlator Intrathoracic Pressure Regulator – Pediatric Version – Product Description 45
Advanced Medical Electronics Corp Pipeline Products & Ongoing Clinical Trials Overview 46
Continuous Pulse Oximeter – Neonatal Monitoring – Product Status 46
Continuous Pulse Oximeter – Neonatal Monitoring – Product Description 46
Aeiyr Inc Pipeline Products & Ongoing Clinical Trials Overview 47
Apnea Monitoring Device – Product Status 47
Apnea Monitoring Device – Product Description 47
Aerobiosys innovations Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 48
J Fit – Product Status 48
J Fit – Product Description 48
Allgene LLC Pipeline Products & Ongoing Clinical Trials Overview 49
SimplyAir Detector – Product Status 49
SimplyAir Detector – Product Description 49
Anaxsys Technology Ltd Pipeline Products & Ongoing Clinical Trials Overview 50
Breathe Easyflow – Product Status 50
Breathe Easyflow – Product Description 50
Sleep Apnea System – Product Status 51
Sleep Apnea System – Product Description 51
Apneon, Inc. Pipeline Products & Ongoing Clinical Trials Overview 52
Apnea Monitor – Product Status 52
Apnea Monitor – Product Description 52
Apnostic LLC Pipeline Products & Ongoing Clinical Trials Overview 53
Diagnostic Device – Sleep Apnea – Product Status 53
Diagnostic Device – Sleep Apnea – Product Description 53
Arizona State University Pipeline Products & Ongoing Clinical Trials Overview 54
Wireless Capnography Device – Product Status 54
Wireless Capnography Device – Product Description 54
Aspire Medical, Inc. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 55
Aspire Medical Advance System – Product Status 55
Aspire Medical Advance System – Product Description 55
Barron Associates, Inc. Pipeline Products & Ongoing Clinical Trials Overview 56
Remote Oxygen Adherence Monitor System – Product Status 56
Remote Oxygen Adherence Monitor System – Product Description 56
SoundTrak System – Product Status 57
SoundTrak System – Product Description 57
Bedfont Scientific Ltd Pipeline Products & Ongoing Clinical Trials Overview 58
Homecare FeNO Device – Product Status 58
Homecare FeNO Device – Product Description 58
Ben-Gurion University of the Negev Pipeline Products & Ongoing Clinical Trials Overview 59
OSA Detection System – Product Status 59
OSA Detection System – Product Description 59
Berendo Scientific LLC Pipeline Products & Ongoing Clinical Trials Overview 60
Natural Sleep Endoscopy System – Product Status 60
Natural Sleep Endoscopy System – Product Description 60
Biospeech Inc Pipeline Products & Ongoing Clinical Trials Overview 61
Sleep Disordered Breathing Detection System – Product Status 61
Sleep Disordered Breathing Detection System – Product Description 61
Bittium Corp Pipeline Products & Ongoing Clinical Trials Overview 62
Bittium Respiro – Product Status 62
Bittium Respiro – Product Description 62
Bodimetrics Pipeline Products & Ongoing Clinical Trials Overview 63
CIRCUL Pulse Oximetry Ring – Product Status 63
CIRCUL Pulse Oximetry Ring – Product Description 63
Briota Technologies Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 64
SpiroPRO – Home Monitoring – Product Status 64
SpiroPRO – Home Monitoring – Product Description 64
Cambridge Consultants Ltd Pipeline Products & Ongoing Clinical Trials Overview 65
Breathe – Product Status 65
Breathe – Product Description 65
Cambridge Respiratory Innovations Ltd Pipeline Products & Ongoing Clinical Trials Overview 66
N-Tidal A – Product Status 66
N-Tidal A – Product Description 66
N-Tidal B – Product Status 67
N-Tidal B – Product Description 67
CARAG AG Pipeline Products & Ongoing Clinical Trials Overview 68
Carag Tissue Oxygenation Monitoring System – Product Status 68
Carag Tissue Oxygenation Monitoring System – Product Description 68
CARAG AG – Ongoing Clinical Trials Overview 69
Carag Tissue Oxygenation Monitoring System – An Open Single-center Study in England to Assess Safety and Performance of a NIRS System to Monitor Abdominal Tissue Oxygen Saturation in Preterm Infants 70
CAS Medical Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 71
OEM Module Combination Device – Product Status 71
OEM Module Combination Device – Product Description 71
Circassia AB Pipeline Products & Ongoing Clinical Trials Overview 72
NIOX VERO Airway Inflammation Monitor – Product Status 72
NIOX VERO Airway Inflammation Monitor – Product Description 72
Circassia AB – Ongoing Clinical Trials Overview 73
NIOX VERO Airway Inflammation Monitor – A Randomized, Multi-center, Single-visit, Clinical Investigation to Assess the Degree of Agreement and Feasibility of Measuring Exhaled Nitric Oxide in Children, Aged 4-6 Years Old, with the NIOX VERO Using the 6 Second Exhalation Mode as Compared with the 10 Second Exhalation Mode 74
NIOX VERO Airway Inflammation Monitor – Improving Diagnostics of Concurrent Inflammatory Airway Diseases in Private ENT Practice – A Study of No Measurements in Screening for Asthma and OSA in Patients with CRSwNP 74
Cirtec Medical Corp Pipeline Products & Ongoing Clinical Trials Overview 75
Pulse Oximetry Device – Product Status 75
Pulse Oximetry Device – Product Description 75
City, University of London Pipeline Products & Ongoing Clinical Trials Overview 76
Ear Canal Pulse Oximetry Probe – Product Status 76
Ear Canal Pulse Oximetry Probe – Product Description 76
Fibre-Optic Sensor – Product Status 77
Fibre-Optic Sensor – Product Description 77
Clairways LLC Pipeline Products & Ongoing Clinical Trials Overview 78
Asthma Monitoring Device – Product Status 78
Asthma Monitoring Device – Product Description 78
PULMO Device – Product Status 79
PULMO Device – Product Description 79
Compumedics Ltd Pipeline Products & Ongoing Clinical Trials Overview 80
Ambulatory Sleep Testing Device – Product Status 80
Ambulatory Sleep Testing Device – Product Description 80
Controle Instrumentation Et Diagnostic Electroniques SA Pipeline Products & Ongoing Clinical Trials Overview 81
PneaVoX Sensor – Product Status 81
PneaVoX Sensor – Product Description 81
Controle Instrumentation Et Diagnostic Electroniques SA – Ongoing Clinical Trials Overview 82
PneaVoX Sensor – Evaluation of a Disposable Sound Sensor for Recording Respiration During Sleep 83
Covidien Ltd Pipeline Products & Ongoing Clinical Trials Overview 84
Pulse Oximeter With Universal Sensor – Product Status 84
Pulse Oximeter With Universal Sensor – Product Description 84
Dartmouth College Pipeline Products & Ongoing Clinical Trials Overview 85
Non-Invasive pO2 Measurement Device – Product Status 85
Non-Invasive pO2 Measurement Device – Product Description 85
Oxygen-Sensing Chip – Product Status 86
Oxygen-Sensing Chip – Product Description 86
EEGSmart Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 87
UMindSleep – Product Status 87
UMindSleep – Product Description 87
EEGSmart Technology Co Ltd – Ongoing Clinical Trials Overview 88
UMindSleep – Validation of a Handy Sleep Monitoring Device: UMindSleep in Patients with Obstructive Sleep Apnea 89
Elecro Chem, Inc. Pipeline Products & Ongoing Clinical Trials Overview 90
Sneezometer – Product Status 90
Sneezometer – Product Description 90
Eupnea AS Pipeline Products & Ongoing Clinical Trials Overview 91
Respiratory Rate Detecting Sensor – Product Status 91
Respiratory Rate Detecting Sensor – Product Description 91
Feather Sensors, LLC Pipeline Products & Ongoing Clinical Trials Overview 92
Breath Gauge – Product Status 92
Breath Gauge – Product Description 92
Florida International University Pipeline Products & Ongoing Clinical Trials Overview 93
Near Infrared Optical Scanner – Product Status 93
Near Infrared Optical Scanner – Product Description 93
Fluorometrix Corp Pipeline Products & Ongoing Clinical Trials Overview 94
tcpO2/tcpCO2 Respiration Monitor – Product Status 94
tcpO2/tcpCO2 Respiration Monitor – Product Description 94
Fraunhofer-Gesellschaft zur Forderung der Angewandten Forschung eV Pipeline Products & Ongoing Clinical Trials Overview 95
Thorax Monitoring System – Product Status 95
Thorax Monitoring System – Product Description 95
GE Global Research Pipeline Products & Ongoing Clinical Trials Overview 96
EIT Lung Function Monitoring System – Product Status 96
EIT Lung Function Monitoring System – Product Description 96
GPX Medical AB Pipeline Products & Ongoing Clinical Trials Overview 97
NEOLA – Product Status 97
NEOLA – Product Description 97
H. Lee Moffitt Cancer Center & Research Institute Inc Pipeline Products & Ongoing Clinical Trials Overview 98
Anesthesia Intra-oral Monitoring System (AIMS) – Product Status 98
Anesthesia Intra-oral Monitoring System (AIMS) – Product Description 98
Health Care Originals Inc Pipeline Products & Ongoing Clinical Trials Overview 99
ADAMM Device – Asthma – Product Status 99
ADAMM Device – Asthma – Product Description 99
Ideaquest Inc Pipeline Products & Ongoing Clinical Trials Overview 100
Adult Respiratory Function Analysis System – Product Status 100
Adult Respiratory Function Analysis System – Product Description 100
Apnea Monitor – Adults – Product Status 101
Apnea Monitor – Adults – Product Description 101
Apnea Monitor – Infants – Product Status 101
Apnea Monitor – Infants – Product Description 102
Infant Respiratory Function Analysis System – Product Status 102
Infant Respiratory Function Analysis System – Product Description 102
Imperial College London Pipeline Products & Ongoing Clinical Trials Overview 103
Afflo – Product Status 103
Afflo – Product Description 103
INCA Medical Systems Ltd Pipeline Products & Ongoing Clinical Trials Overview 104
Ventilator FRC – Product Status 104
Ventilator FRC – Product Description 104
Indian Institute of Technology Delhi Pipeline Products & Ongoing Clinical Trials Overview 105
Spo2 Measurement Device – Product Status 105
Spo2 Measurement Device – Product Description 105
Inofab Health Technologies Pipeline Products & Ongoing Clinical Trials Overview 106
Spirohome Personal – Product Status 106
Spirohome Personal – Product Description 106
Intelligent Fiber Optic Systems Corporation Pipeline Products & Ongoing Clinical Trials Overview 107
IFOS OSA Catheter – Product Status 107
IFOS OSA Catheter – Product Description 107
Intelligent Optical Systems, Inc. Pipeline Products & Ongoing Clinical Trials Overview 108
Integrated Fiber Optic Sensor Umbilical Catheter – Product Status 108
Integrated Fiber Optic Sensor Umbilical Catheter – Product Description 108
Pulse Oximeter For Newborn Screening – Product Status 109
Pulse Oximeter For Newborn Screening – Product Description 109
ISS Inc Pipeline Products & Ongoing Clinical Trials Overview 110
METAOX – Product Status 110
METAOX – Product Description 110
ItoM Medical BV Pipeline Products & Ongoing Clinical Trials Overview 111
Wearable Smart Vest Device – Asthma – Product Status 111
Wearable Smart Vest Device – Asthma – Product Description 111
Johns Hopkins University Pipeline Products & Ongoing Clinical Trials Overview 112
Lyra At-Home PSG – Product Status 112
Lyra At-Home PSG – Product Description 112
K-diagnostics Pipeline Products & Ongoing Clinical Trials Overview 113
Sleep ID Monitor – Product Status 113
Sleep ID Monitor – Product Description 113
Kestrel Labs Inc Pipeline Products & Ongoing Clinical Trials Overview 114
NiCO CO-Oximeter – Product Status 114
NiCO CO-Oximeter – Product Description 114
Koronis Biomedical Technologies Corporation Pipeline Products & Ongoing Clinical Trials Overview 115
FOT Device – Product Status 115
FOT Device – Product Description 115
Home-Based Spirometer Device – Product Status 116
Home-Based Spirometer Device – Product Description 116
Low Power Wireless Pulse Oximeter Sensor – Product Status 116
Low Power Wireless Pulse Oximeter Sensor – Product Description 117
Ultrasonic Spirometer – Home Monitoring Of Asthma – Product Status 117
Ultrasonic Spirometer – Home Monitoring Of Asthma – Product Description 117
Laser Associated Sciences, Inc. Pipeline Products & Ongoing Clinical Trials Overview 118
Flowmetry-based Pulse Oximeter – Product Status 118
Flowmetry-based Pulse Oximeter – Product Description 118
Lifewave Biomedical Inc Pipeline Products & Ongoing Clinical Trials Overview 119
CardioConnect – Pulmonary Edema – Product Status 119
CardioConnect – Pulmonary Edema – Product Description 119
CardioConnect – Respiration Monitoring – Product Status 120
CardioConnect – Respiration Monitoring – Product Description 120
CardioConnect – Sleep Apnea – Product Status 120
CardioConnect – Sleep Apnea – Product Description 121
Lionsgate Technologies Inc. Pipeline Products & Ongoing Clinical Trials Overview 122
Pneumonia Kit – Product Status 122
Pneumonia Kit – Product Description 122
Lundquist Pipeline Products & Ongoing Clinical Trials Overview 123
Fiber Optic Sensor Umbilical Catheter – Product Status 123
Fiber Optic Sensor Umbilical Catheter – Product Description 123
Mad Pow Media Solutions, LLC Pipeline Products & Ongoing Clinical Trials Overview 124
Aspira Asthma Home Monitoring System – Product Status 124
Aspira Asthma Home Monitoring System – Product Description 124
Makani Science Inc Pipeline Products & Ongoing Clinical Trials Overview 125
Makani Respiration Monitor – Product Status 125
Makani Respiration Monitor – Product Description 125
Masimo Corp Pipeline Products & Ongoing Clinical Trials Overview 126
iSpO2 Rx – Product Status 126
iSpO2 Rx – Product Description 127
Masimo Rainbow SET – SpfO2 – Product Status 127
Masimo Rainbow SET – SpfO2 – Product Description 127
Masimo Rainbow SET Pulse CO-Oximetry – RPVi – Product Status 128
Masimo Rainbow SET Pulse CO-Oximetry – RPVi – Product Description 128
Masimo Rainbow SET Pulse CO-Oximetry – RRp – Product Status 128
Masimo Rainbow SET Pulse CO-Oximetry – RRp – Product Description 129
Masimo SET – Opioid Overdose – Product Status 129
Masimo SET – Opioid Overdose – Product Description 129
Rad-G With Temperature – Product Status 130
Rad-G With Temperature – Product Description 130
rainbow Super DCI-mini sensor – Continuous Measurement Version – Product Status 130
rainbow Super DCI-mini sensor – Continuous Measurement Version – Product Description 131
Rainbow SuperSensor – Product Status 131
Rainbow SuperSensor – Product Description 131
Med-Botics LLC Pipeline Products & Ongoing Clinical Trials Overview 132
Oxalert EPO – Product Status 132
Oxalert EPO – Product Description 132
Med-Botics LLC – Ongoing Clinical Trials Overview 133
Oxalert EPO – Wearable Device for Prevention of Opioid-induced Hypoxemia 134
Medipines Corp Pipeline Products & Ongoing Clinical Trials Overview 135
MediPines Gas Exchange Monitor AGM100 – Pediatric – Product Status 135
MediPines Gas Exchange Monitor AGM100 – Pediatric – Product Description 135
Oxistimulator Device – Product Status 136
Oxistimulator Device – Product Description 136
Medtronic Plc Pipeline Products & Ongoing Clinical Trials Overview 137
Capnostream Monitor – Opioid-Induced Respiratory Depression – Product Status 137
Capnostream Monitor – Opioid-Induced Respiratory Depression – Product Description 138
Nellcor OxySoft Neonatal Sensor – Product Status 138
Nellcor OxySoft Neonatal Sensor – Product Description 138
Nanomix Inc Pipeline Products & Ongoing Clinical Trials Overview 139
CapLite – Product Status 139
CapLite – Product Description 139
Nanomix Asthma Management System – Product Status 140
Nanomix Asthma Management System – Product Description 140
NanoVation-GS Pipeline Products & Ongoing Clinical Trials Overview 141
Stick-on Patch – Product Status 141
Stick-on Patch – Product Description 141
NE Field Diagnostics Ltd Pipeline Products & Ongoing Clinical Trials Overview 142
A-Spire – Product Status 142
A-Spire – Product Description 142
Neetour Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 143
Capno-Pulse – Product Status 143
Capno-Pulse – Product Description 143
Neogia SAS Pipeline Products & Ongoing Clinical Trials Overview 144
Motio HW – Product Status 144
Motio HW – Product Description 144
Neovative Inc Pipeline Products & Ongoing Clinical Trials Overview 145
Asthma Gram Patch – Product Status 145
Asthma Gram Patch – Product Description 145
Nicrem S.r.l. Pipeline Products & Ongoing Clinical Trials Overview 146
NICRem Baby – Product Status 146
NICRem Baby – Product Description 146
Respiratory Holter – COPD – Product Status 147
Respiratory Holter – COPD – Product Description 147
Respiratory Holter – Heart Disease – Product Status 147
Respiratory Holter – Heart Disease – Product Description 148
SERIES 100 – Product Status 148
SERIES 100 – Product Description 148
Nihon Kohden Corp Pipeline Products & Ongoing Clinical Trials Overview 149
OLV-4202 Pulse Oximeter – Product Status 149
OLV-4202 Pulse Oximeter – Product Description 149
Nihon Kohden Corp – Ongoing Clinical Trials Overview 150
OLV-4202 Pulse Oximeter – Accuracy of Pulse Oximeters with Profound Hypoxia NIHO 14 151
OLV-4202 Pulse Oximeter – Determination of SpO2 and PR Accuracy Specifications at Rest Accuracy of Pulse Oximeters with Profound Hypoxia Pulse Oximeter Accuracy Evaluation Protocol 151
Noninvasix Inc Pipeline Products & Ongoing Clinical Trials Overview 152
LIVOx – Product Status 152
LIVOx – Product Description 152
Onera BV Pipeline Products & Ongoing Clinical Trials Overview 153
Onera Sleep Test System – Product Status 153
Onera Sleep Test System – Product Description 153
Onera BV – Ongoing Clinical Trials Overview 154
Onera Sleep Test System – Study Evaluating the Diagnostic Accuracy of Onera STS I in Comparison to in-lab Polysomnography 155
Onera Sleep Test System – Validation Study of a Patch-based PSG System 155
Opticyte Inc Pipeline Products & Ongoing Clinical Trials Overview 156
CellSat-100 – Product Status 156
CellSat-100 – Product Description 156
Oregon Health & Science University Pipeline Products & Ongoing Clinical Trials Overview 157
Non-Obtrusive System – Sleep Disorders – Product Status 157
Non-Obtrusive System – Sleep Disorders – Product Description 157
Owlet Baby Care Inc Pipeline Products & Ongoing Clinical Trials Overview 158
Owlet BabySat Sensor – Product Status 158
Owlet BabySat Sensor – Product Description 158
Owlet Smart Sock – Product Status 159
Owlet Smart Sock – Product Description 159
Pendar Technologies LLC Pipeline Products & Ongoing Clinical Trials Overview 160
Microvascular Oximeter – Product Status 160
Microvascular Oximeter – Product Description 160
PMD Device Solutions Ltd Pipeline Products & Ongoing Clinical Trials Overview 161
RespiraSense – Product Status 161
RespiraSense – Product Description 161
Pneumedicare Ltd. Pipeline Products & Ongoing Clinical Trials Overview 162
Pneumonitor – Product Status 162
Pneumonitor – Product Description 162
Pneumonics Inc Pipeline Products & Ongoing Clinical Trials Overview 163
PneuMotion – Product Status 163
PneuMotion – Product Description 163
Polytechnique Montreal Pipeline Products & Ongoing Clinical Trials Overview 164
Microsystem – Respiratory Parameters – Product Status 164
Microsystem – Respiratory Parameters – Product Description 164
Pumani Pipeline Products & Ongoing Clinical Trials Overview 165
BabaLung Apnea Monitor – Product Status 165
BabaLung Apnea Monitor – Product Description 165
Raydiant Oximetry Inc Pipeline Products & Ongoing Clinical Trials Overview 166
Trans-Abdominal Fetal Oximeter – Product Status 166
Trans-Abdominal Fetal Oximeter – Product Description 166
ResApp Health Ltd Pipeline Products & Ongoing Clinical Trials Overview 167
Handheld Breathing Monitor – Product Status 167
Handheld Breathing Monitor – Product Description 167
ResMed Inc Pipeline Products & Ongoing Clinical Trials Overview 168
Leo Device – Product Status 168
Leo Device – Product Description 168
Masksense – Product Status 169
Masksense – Product Description 169
ResMed Inc – Ongoing Clinical Trials Overview 170
Leo Device – Engineering Validation of Leo Device to Assess Clinical Control of Children Recovering From Acute Asthma Exacerbation 171
RespiNor AS Pipeline Products & Ongoing Clinical Trials Overview 172
RESPINOR DXT – Product Status 172
RESPINOR DXT – Product Description 172
RespiNor AS – Ongoing Clinical Trials Overview 173
RESPINOR DXT – Multicenter, Multinational, Clinical Trial of the Performance of RESPINOR DXT to Identify Patients at Increased Risk of Weaning Failure 174
RESPINOR DXT – Phase II Clinical Study to Evaluate the Effectiveness of DiaMon in Monitoring of the Diaphragm Function in Subjects on Ventilators 174
Respira Ltd Pipeline Products & Ongoing Clinical Trials Overview 175
Digital Peak Flow Meter – Product Status 175
Digital Peak Flow Meter – Product Description 175
Digital Snoring Meter – Product Status 176
Digital Snoring Meter – Product Description 176
PulmoTrack 3010 CC – Product Status 176
PulmoTrack 3010 CC – Product Description 177
WIM-GER – Product Status 177
WIM-GER – Product Description 177
Respiratory Motion Inc Pipeline Products & Ongoing Clinical Trials Overview 178
ExSpiron – Newborns And Premature Infants – Product Status 178
ExSpiron – Newborns And Premature Infants – Product Description 178
Respiri Ltd Pipeline Products & Ongoing Clinical Trials Overview 179
Rapid Daytime Test – OSA – Product Status 179
Rapid Daytime Test – OSA – Product Description 179
wheezo – Product Status 180
wheezo – Product Description 180
Respiri Ltd – Ongoing Clinical Trials Overview 181
wheezo – A Clinical Study to Evaluate the Efficacy of Wheezo’s Acoustic Respiratory Monitoring in Patients with an Exacerbation of Airway Disease 182
wheezo – Clinical Study Examine the Measurement of wheeze For Small Airway Function (Asthma) 182
Rice University Pipeline Products & Ongoing Clinical Trials Overview 183
Luminox – Product Status 183
Luminox – Product Description 183
Rostrum Medical Innovations Inc Pipeline Products & Ongoing Clinical Trials Overview 184
VQm Pulmonary Health Monitor – Product Status 184
VQm Pulmonary Health Monitor – Product Description 184
RS Medical Monitoring Ltd. Pipeline Products & Ongoing Clinical Trials Overview 185
Edema Guard Monitor – Product Status 185
Edema Guard Monitor – Product Description 185
RTM Vital Signs LLC Pipeline Products & Ongoing Clinical Trials Overview 186
Acoustic Ventilation Device – Product Status 186
Acoustic Ventilation Device – Product Description 186
RTM Vital Signs LLC – Ongoing Clinical Trials Overview 187
Acoustic Ventilation Device – Development of an Algorithm that Predicts Hypoventilation Due to an Opioid Overdose 188
Safe Heart USA Inc Pipeline Products & Ongoing Clinical Trials Overview 189
Pediatric iOximeter – Product Status 189
Pediatric iOximeter – Product Description 189
Sheffield Hallam University Pipeline Products & Ongoing Clinical Trials Overview 190
4FA Respiration Monitor – Product Status 190
4FA Respiration Monitor – Product Description 190
Sleepiz AG Pipeline Products & Ongoing Clinical Trials Overview 191
Sleepiz One – Product Status 191
Sleepiz One – Product Description 191
Sleepiz One Connect – Product Status 192
Sleepiz One Connect – Product Description 192
Sleepiz AG – Ongoing Clinical Trials Overview 193
Sleepiz One – Identification of Patients with Clinically Relevant Sleep Apnea with a Contactless Device in Rehabilitation Clinic 194
Sleepiz One Connect – Recording of Multiple Nights Using a New Contactless Device (Sleepiz One Connect) in Obstructive Sleep Apnea 195
SleepUp Ltd Pipeline Products & Ongoing Clinical Trials Overview 196
AICOT – Product Status 196
AICOT – Product Description 196
AirThrough Pacifier – Product Status 197
AirThrough Pacifier – Product Description 197
STEMA – Product Status 197
STEMA – Product Description 197
Sonosa Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 198
Wearable Ultrasound System – Product Status 198
Wearable Ultrasound System – Product Description 198
Spircare (2016) Ltd Pipeline Products & Ongoing Clinical Trials Overview 199
Spircare Device – Product Status 199
Spircare Device – Product Description 199
SPIROCHIP Pipeline Products & Ongoing Clinical Trials Overview 200
Intelligent Personal Spirometer and Inflammometer – Product Status 200
Intelligent Personal Spirometer and Inflammometer – Product Description 200
SRETT SAS Pipeline Products & Ongoing Clinical Trials Overview 201
TeleOx – Product Status 201
TeleOx – Product Description 201
Stellenbosch University Pipeline Products & Ongoing Clinical Trials Overview 202
Artificial Pulse Oximeter – Product Status 202
Artificial Pulse Oximeter – Product Description 202
Flow Volume Meter – Product Status 203
Flow Volume Meter – Product Description 203
Mobile Spirometer – Product Status 203
Mobile Spirometer – Product Description 204
Sunrise SA Pipeline Products & Ongoing Clinical Trials Overview 205
Sunrise – Product Status 205
Sunrise – Product Description 205
Sunrise SA – Ongoing Clinical Trials Overview 206
Sunrise – A Prospective, Multicentre, Randomised, Blinded Study of Obstructive Sleep Apnoea Detection Using the Sunrise Solution 207
Sunrise – Randomized Controlled Trial of Transcutaneous Electrical Stimulation in Obstructive Sleep Apnea 207
Sunrise – Validation of an Integrated Digital Solution (Sunrise) for Automatic Analysis of Mandibular Movements by Artificial Intelligence Versus Polysomnography for the Diagnosis of Obstructive Sleep Apnea Syndrome 207
Sunrise SPRL Pipeline Products & Ongoing Clinical Trials Overview 208
At-Home Sleep Apnea Device – Product Status 208
At-Home Sleep Apnea Device – Product Description 208
TessArae, LLC Pipeline Products & Ongoing Clinical Trials Overview 209
SONOSA System – Product Status 209
SONOSA System – Product Description 209
TF Health Corporation Pipeline Products & Ongoing Clinical Trials Overview 210
Mobile Multifunctional Capnography And Spirometry Device – Product Status 210
Mobile Multifunctional Capnography And Spirometry Device – Product Description 210
The Royal Children’s Hospital Pipeline Products & Ongoing Clinical Trials Overview 211
Lung Function Monitoring Device – Product Status 211
Lung Function Monitoring Device – Product Description 211
The University of British Columbia Pipeline Products & Ongoing Clinical Trials Overview 212
Camera Oximeter – Product Status 212
Camera Oximeter – Product Description 212
Tufts University Pipeline Products & Ongoing Clinical Trials Overview 213
Noninvasive Absolute Brain Tissue Oximeter – Product Status 213
Noninvasive Absolute Brain Tissue Oximeter – Product Description 213
TytoCare Ltd Pipeline Products & Ongoing Clinical Trials Overview 214
TytoCare SpO2 Device – Product Status 214
TytoCare SpO2 Device – Product Description 214
University of California Pipeline Products & Ongoing Clinical Trials Overview 215
Portable Lung Function Monitor – Product Status 215
Portable Lung Function Monitor – Product Description 215
University of California Berkeley Pipeline Products & Ongoing Clinical Trials Overview 216
Pulse Oximeter Sensor – Product Status 216
Pulse Oximeter Sensor – Product Description 216
University of California Irvine Pipeline Products & Ongoing Clinical Trials Overview 217
Upper Airway Imaging Device – Product Status 217
Upper Airway Imaging Device – Product Description 217
Wearable Respiration Monitor – Product Status 218
Wearable Respiration Monitor – Product Description 218
University of California Los Angeles Pipeline Products & Ongoing Clinical Trials Overview 219
Pressure Sensor System – Product Status 219
Pressure Sensor System – Product Description 219
University of California San Diego Pipeline Products & Ongoing Clinical Trials Overview 220
Portable Hand Held Alveolar Oxygen And Carbon Dioxide Meter – Product Status 220
Portable Hand Held Alveolar Oxygen And Carbon Dioxide Meter – Product Description 220
University of Colorado Pipeline Products & Ongoing Clinical Trials Overview 221
Hypoxemia Device – Product Status 221
Hypoxemia Device – Product Description 221
University of Eastern Finland Pipeline Products & Ongoing Clinical Trials Overview 222
Neural Network – Product Status 222
Neural Network – Product Description 222
University of Illinois at Chicago Pipeline Products & Ongoing Clinical Trials Overview 223
Large Memory Storage And Retrieval Neural Network Device – Product Status 223
Large Memory Storage And Retrieval Neural Network Device – Product Description 223
University of Massachusetts Amherst Pipeline Products & Ongoing Clinical Trials Overview 224
Foam Sleeping Mask – Product Status 224
Foam Sleeping Mask – Product Description 224
University of Nebraska Omaha Pipeline Products & Ongoing Clinical Trials Overview 225
COPD Detection Platform – Product Status 225
COPD Detection Platform – Product Description 225
University of Oxford Pipeline Products & Ongoing Clinical Trials Overview 226
Breath-By-Breath Respiratory Gas Analyser – Product Status 226
Breath-By-Breath Respiratory Gas Analyser – Product Description 226
University of Pennsylvania Pipeline Products & Ongoing Clinical Trials Overview 227
InSync Device – Respiratory Distress – Product Status 227
InSync Device – Respiratory Distress – Product Description 227
University of Surrey Pipeline Products & Ongoing Clinical Trials Overview 228
Respirometer – Product Status 228
Respirometer – Product Description 228
University of Texas at Arlington Pipeline Products & Ongoing Clinical Trials Overview 229
Sleep Disorder Breathing Detection System – Product Status 229
Sleep Disorder Breathing Detection System – Product Description 229
University of Toulon Pipeline Products & Ongoing Clinical Trials Overview 230
Sleep Apnea Contactless Device – Product Status 230
Sleep Apnea Contactless Device – Product Description 230
University of Vermont Pipeline Products & Ongoing Clinical Trials Overview 231
Disposable Impedance Adaptor (DIA) – Product Status 231
Disposable Impedance Adaptor (DIA) – Product Description 231
University of Washington Pipeline Products & Ongoing Clinical Trials Overview 232
SpiroSmart – Product Status 232
SpiroSmart – Product Description 232
Uscom Ltd Pipeline Products & Ongoing Clinical Trials Overview 233
SpiroReader – Product Status 233
SpiroReader – Product Description 233
SpiroSonic AIR – Product Status 234
SpiroSonic AIR – Product Description 234
SpiroSonic HD – Product Status 234
SpiroSonic HD – Product Description 235
Spirosonic Whistler – Product Status 235
Spirosonic Whistler – Product Description 235
Spirosonic WiFi – Product Status 236
Spirosonic WiFi – Product Description 236
VA Pittsburgh Healthcare System Pipeline Products & Ongoing Clinical Trials Overview 237
Heated Pulse-Oximeter Probe – Product Status 237
Heated Pulse-Oximeter Probe – Product Description 237
Vapotherm Inc Pipeline Products & Ongoing Clinical Trials Overview 238
Precision Flow System – IntellO2 Module – Product Status 238
Precision Flow System – IntellO2 Module – Product Description 239
Vapotherm Inc – Ongoing Clinical Trials Overview 240
Precision Flow System – IntellO2 Module – SCO2T Study: A Randomised Crossover Study Comparing Pulse Oximeter Technology Using Automatic Oxygen Control for Preterm Infants 241
Precision Flow System – IntellO2 Module – Servo Controlled Oxygen Targeting (SCO2T): Servo Versus Servo 241
Vigor Medical Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 242
Mobile Spirometer – Product Status 242
Mobile Spirometer – Product Description 242
VSSB Medical Nanotechnology (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 243
VX100 – Product Status 243
VX100 – Product Description 243
VX200 – Product Status 244
VX200 – Product Description 244
Wipox LLC Pipeline Products & Ongoing Clinical Trials Overview 245
WiPOX – Product Status 245
WiPOX – Product Description 245
Worcester Polytechnic Institute Pipeline Products & Ongoing Clinical Trials Overview 246
Wearable Oxygen Monitor – Adults – Product Status 246
Wearable Oxygen Monitor – Adults – Product Description 246
Wearable Oxygen Monitor – Infants – Product Status 247
Wearable Oxygen Monitor – Infants – Product Description 247
Xhale Inc Pipeline Products & Ongoing Clinical Trials Overview 248
Respiratory Oximeter – Product Status 248
Respiratory Oximeter – Product Description 248
Zansors LLC Pipeline Products & Ongoing Clinical Trials Overview 249
Zerify – Product Status 249
Zerify – Product Description 249
Glossary 367
![]()