Dialysis Accessories Pipeline Report including Stages of Development, Segments, Region and Countries, Regulatory Path and Key Companies,2022 Update
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Dialysis accessories are the accessories that are used along with the machines in renal dialysis procedures. The dialysis accessories pipeline market research report provides comprehensive information about the dialysis accessories pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials which are in progress. Moreover, the report provides information about various pipeline products and their estimated approval dates.
Dialysis Accessories Pipeline Products Market Segments
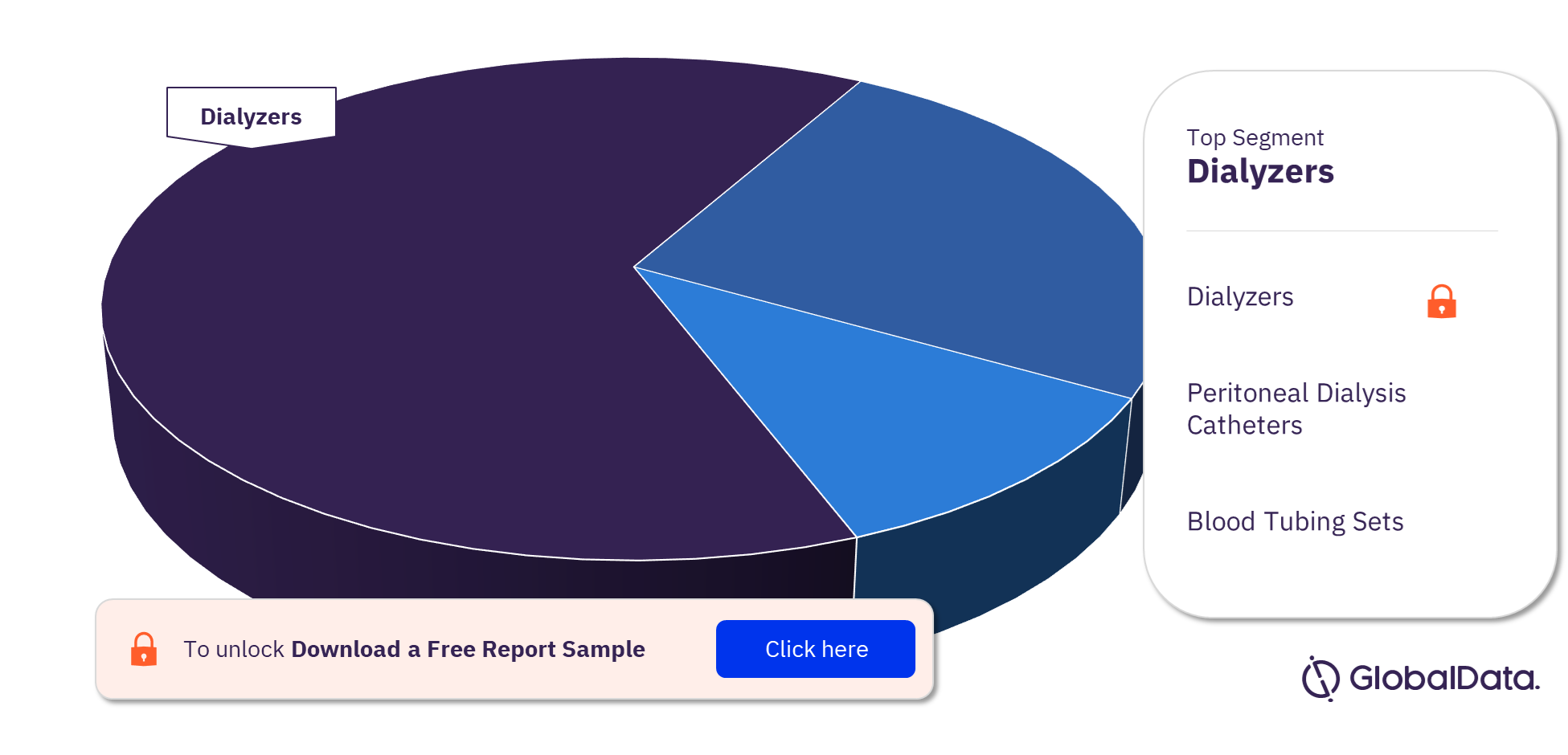
The key segments in the dialysis accessories pipeline products market are dialyzers, peritoneal dialysis catheters, and blood tubing sets. Dialyzers had the highest number of pipeline products in the dialysis accessories pipeline products market.
Dialyzers: Dialyzers are special filters used in hemodialysis and continuous renal replacement therapy for removing toxic substances and an excess of water from the blood. One unit consists of one dialyzer.
Peritoneal Dialysis Catheters: These are soft catheters that are used in peritoneal dialysis to carry the dialysis solution into and out of the abdomen. The standard catheter for peritoneal dialysis is made of soft tubing which has polyester cuffs. One unit refers to one peritoneal dialysis catheter
Blood Tubing Sets: Blood tubing sets act as the connecting link between the blood vessels and the dialysis machine via the AV fistula needles. They are used to carry blood to and from the body. One unit consists of a pair of blood tubing sets.
Dialysis Accessories Pipeline Products Market Analysis by Segments
For more segment insights into the dialysis accessories pipeline products market, download a free report sample
Dialysis Accessories Pipeline Products Market Segmentation by Territories
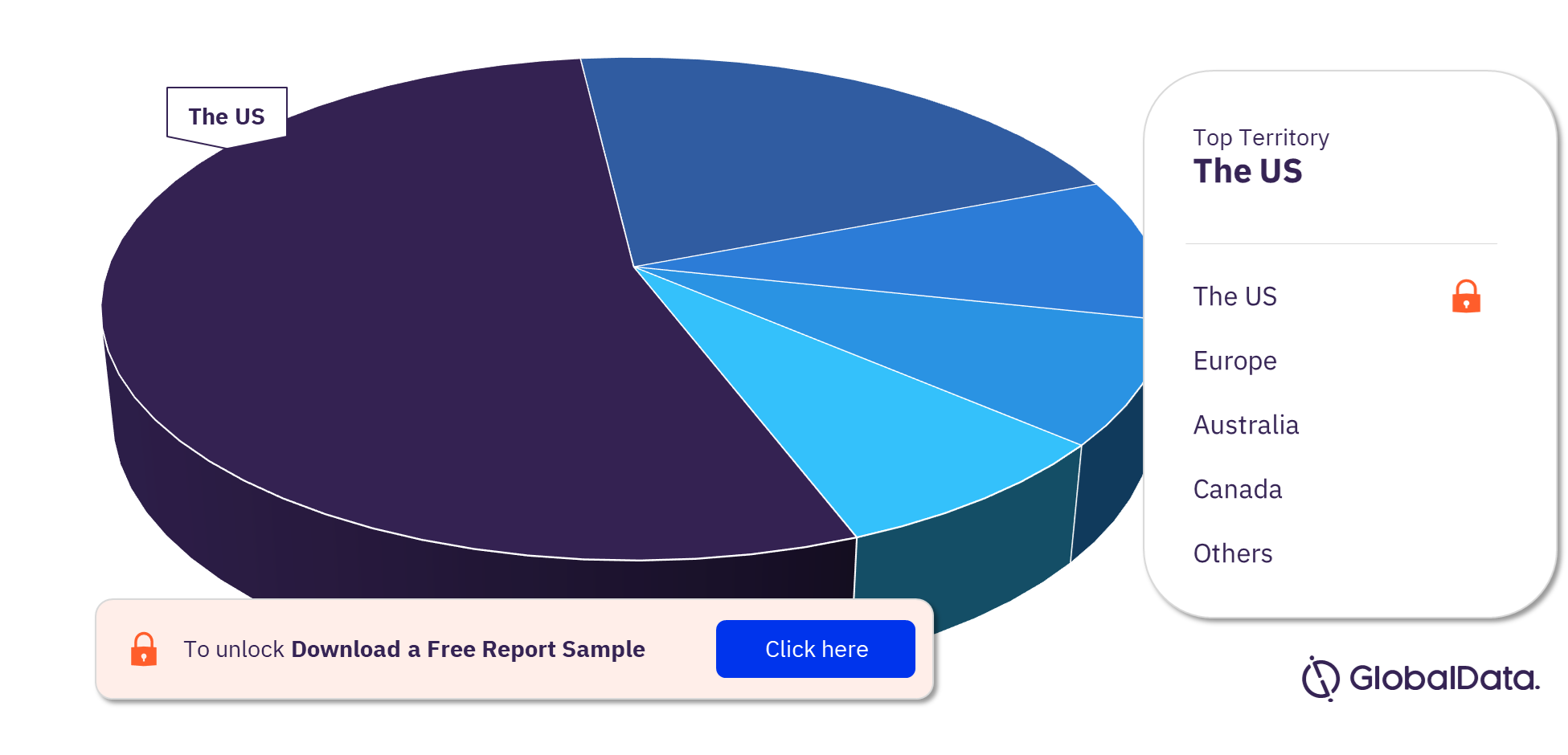
Some of the key territories with products in the pipeline are the US, Europe, Australia, Canada, China, Israel, Italy, South Africa, United Arab Emirates, Bermuda, and Brazil. The US is the leading territory in the dialysis accessories pipeline products market followed by Europe and Australia.
Dialysis Accessories Pipeline Products Market Analysis by Territories
For more territory insights into the dialysis accessories pipeline products market, download a free report sample
Dialysis Accessories Pipeline Products Market Segmentation by Regulatory Paths
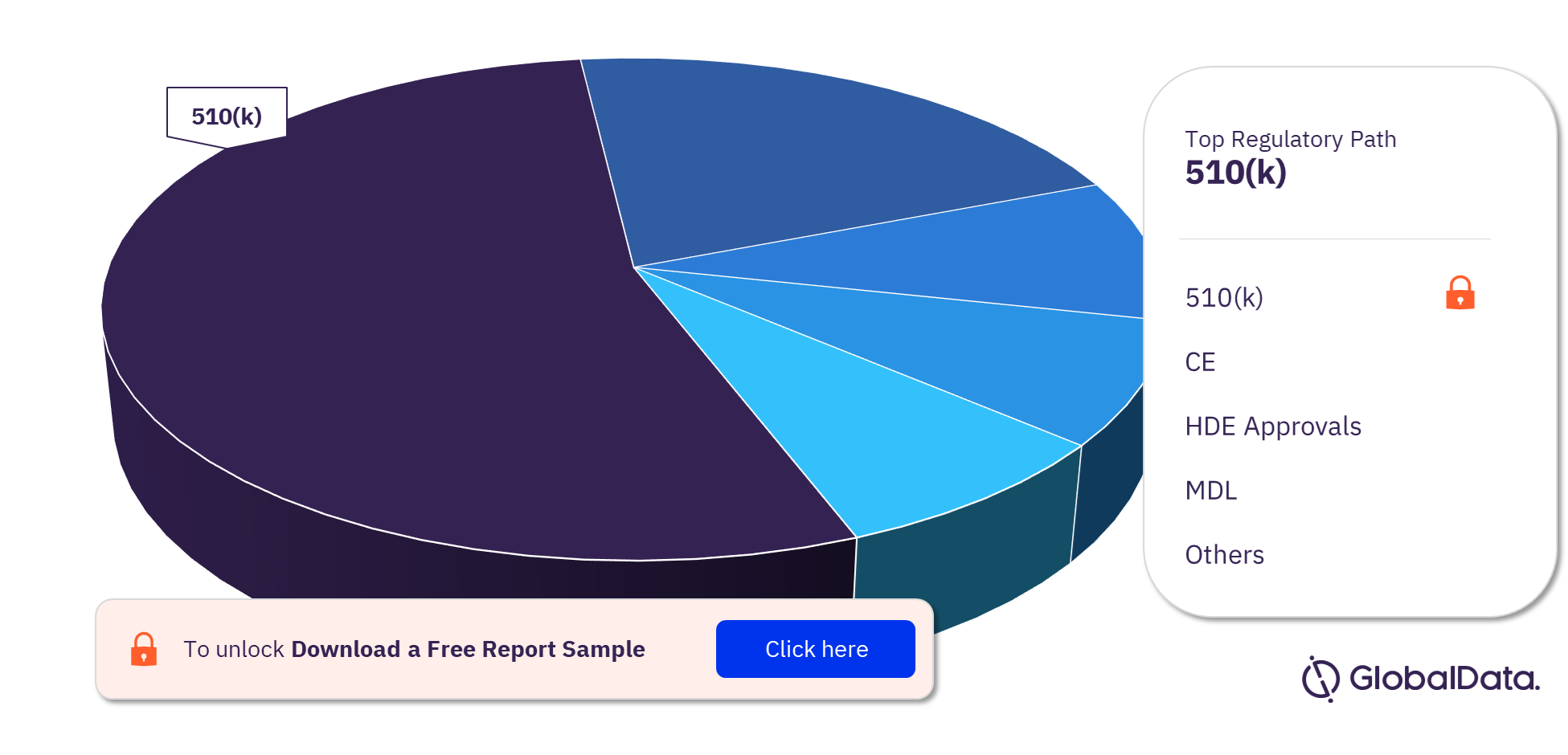
The dialysis accessories pipeline report provides detailed insights into the pipeline products by a regulatory path including 510(k), CE, HDE Approvals, MDL, NMPA, TGA, PMA, ICAC, de novo, and ANVISA. Most of the products follow the 510(k) pathway to enter the market.
Dialysis Accessories Pipeline Products Market Analysis by Regulatory Paths
For more regulatory path insights into the dialysis accessories pipeline products market, download a free report sample
Dialysis Accessories Pipeline Products Market: Competitive Landscape
Some of the leading companies in the dialysis accessories pipeline products market are Access Vascular Inc, Ash Access Technology Inc, Baxter International Inc, Brookhaven Medical Inc, CatheNect Medical, Cerovations LLC, Diavantis, Eqalix Inc., ExThera Medical Corp, Fistulink Ltd., Fresenius Medical Care AG & Co KGaA, and Gambro Dialysatoren GmbH.
Dialysis Accessories Pipeline Products Market Report Overview
| Key Territories | The US, Europe, Australia, Canada, China, Israel, Italy, South Africa, United Arab Emirates, Bermuda, and Brazil |
| Key Regulatory Paths | 510(k), CE, HDE Approvals, MDL, NMPA, TGA, PMA, ICAC, de novo, and ANVISA |
| Key Segments | Dialyzers, Peritoneal Dialysis Catheters, and Blood Tubing Sets |
| Leading Companies | Access Vascular Inc, Ash Access Technology Inc, Baxter International Inc, Brookhaven Medical Inc, CatheNect Medical, Cerovations LLC, Diavantis, Eqalix Inc., ExThera Medical Corp, Fistulink Ltd., Fresenius Medical Care AG & Co KGaA, and Gambro Dialysatoren GmbH |
Scope
- Extensive coverage of the dialysis accessories under development
- The report reviews details of major pipeline products which includes, product description, licensing and collaboration details, and other developmental activities
- The report reviews the major players involved in the development of dialysis accessories and lists all their pipeline projects
- The coverage of pipeline products is based on various stages of development ranging from early development to the approved/issued stage
- The report provides key clinical trial data of ongoing trials specific to pipeline products
- Recent developments in the segment/industry
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies
- Identify emerging players with potentially strong product portfolios and create effective counter strategies to gain a competitive advantage
- Identify and understand important and diverse types of dialysis accessories under development
- Develop market-entry and market-expansion strategies
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date
Ash Access Technology Inc
Baxter International Inc
Brookhaven Medical Inc
CatheNect Medical
Cerovations LLC
Diavantis
Eqalix, Inc.
ExThera Medical Corp
Fistulink Ltd.
Fresenius Medical Care AG & Co KGaA
Gambro Dialysatoren GmbH
Georgia Institute of Technology
Glomeria Therapeutics srl
Healionics Corporation
Indian Institute of Technology Bombay
Innovative BioTherapies Inc
Katharos, Inc.
Laminate Medical Technologies Ltd
Nikkiso Co Ltd
Nipro Corp
Northwestern University
Novaflux Technologies
NuVascular Technologies Inc
Pursuit Vascular, Inc
Rajamadhangi Medical Technologies Pvt Ltd
Redsense Medical AB
Renal Solutions Inc
SeaStar Medical
Silmag SA
Simergent LLC
Specialty Renal Products Inc
University of California San Francisco
University of Michigan
University of Rochester
University of Vermont
Vital Access Corp
Voyager Biomedical Inc
Table of Contents
Table
Figures
Frequently asked questions
-
Which are the key territories in the dialysis accessories pipeline products market?
The key territories in the dialysis accessories pipeline products market are the US, Europe, Australia, Canada, China, Israel, Italy, South Africa, United Arab Emirates, Bermuda, and Brazil.
-
What are the key regulatory paths in the dialysis accessories pipeline products market?
The key regulatory paths in the dialysis accessories pipeline products market are 510(k), CE, HDE Approvals, MDL, NMPA, TGA, PMA, ICAC, de novo, and ANVISA.
-
What are the key segments in the dialysis accessories pipeline products market?
The key segments in the dialysis accessories pipeline products market are dialyzers, peritoneal dialysis catheters, and blood tubing sets.
-
Which are the leading companies in the dialysis accessories pipeline products market?
Some of the leading companies in the dialysis accessories pipeline products market are Access Vascular Inc, Ash Access Technology Inc, Baxter International Inc, Brookhaven Medical Inc, CatheNect Medical, Cerovations LLC, Diavantis, Eqalix Inc., ExThera Medical Corp, Fistulink Ltd., Fresenius Medical Care AG & Co KGaA, and Gambro Dialysatoren GmbH.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Nephrology and Urology Devices reports