Dystrophin (DMD) Drugs in Development by Therapy Areas and Indications, Stages, MoA, RoA, Molecule Type and Key Players, 2022 Update
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Dystrophin Pipeline Drugs Market Report Overview
Dystrophin is a cytoplasmic protein, which anchors the extracellular matrix to the cytoskeleton via F-actin. It acts as ligand for dystroglycan. It also acts as component of the dystrophin-associated glycoprotein complex which accumulates at the neuromuscular junction and at a variety of synapses in the peripheral and central nervous systems and has a structural function in stabilizing the sarcolemma.
The Dystrophin – Drugs in Development research report provides a comprehensive overview on the therapeutics under development for Dystrophin, complete with analysis by stage of development, drug target, mechanism of action (MoA), route of administration (RoA) and molecule type. The report also covers the descriptive pharmacological action of the therapeutics, its complete research and development history and latest news and press releases. Additionally, the report provides an overview of key players involved in therapeutic development for Dystrophin and features dormant and discontinued projects.
| Key Therapy Areas | Genetic Disorders, and Musculoskeletal Disorders |
| Key Mechanisms of action | Dystrophin Activator |
| Key Routes of Administration | Intravenous, Subcutaneous, Intramuscular, and Parenteral |
| Key molecule types | Antisense Oligonucleotide, Gene Therapy, Cell Therapy, Antisense Gene Therapy, Monoclonal Antibody Conjugated, Gene-Modified Cell Therapy, Oligonucleotide, and Protein |
| Major companies | Sarepta Therapeutics Inc, Astellas Gene Therapies, Avidity Biosciences Inc, Daiichi Sankyo Co Ltd, Dystrogen Therapeutics SA, Editas Medicine Inc, Nippon Shinyaku Co Ltd, NS Pharma Inc, OliPass Corporation, Pepgen Ltd, Pfizer Inc, and RegenxBio Inc |
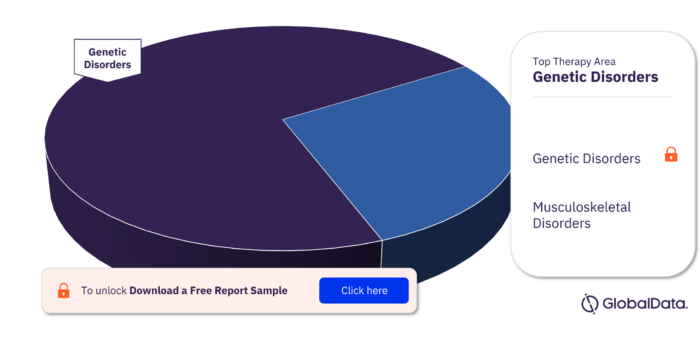
Key Therapy Areas in the Dystrophin Pipeline Drugs Market
In the Dystrophin pipeline drugs market the key therapy areas are Genetic Disorders and Musculoskeletal Disorders. Genetic Disorders led the market in 2022.
Dystrophin Pipeline Drugs Market, by Therapy Areas, 2022 (%)
For more therapy area insights, download a free report sample
Key MoA in the Dystrophin Pipeline Drugs Market
In the Dystrophin pipeline drugs market the key MoA is Dystrophin Activator.
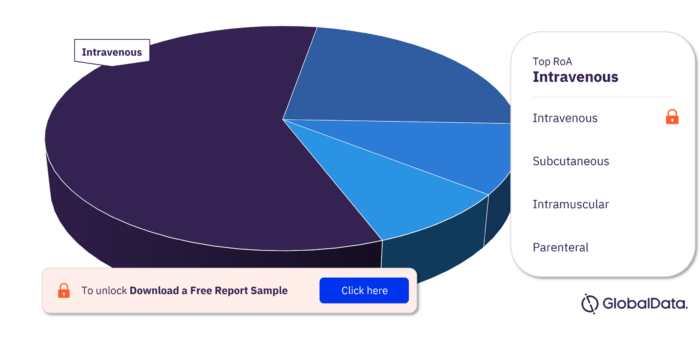
Dystrophin Pipeline Drugs Market Segmentation by RoA
The key routes of administration in the Dystrophin pipeline drugs market are intravenous, subcutaneous, intramuscular, and parenteral. Intravenous has the maximum number of pipeline products in 2022.
Dystrophin Pipeline Drugs Market Analysis, by RoA, 2022 (%)
To get more insights on key RoA, download a free sample report
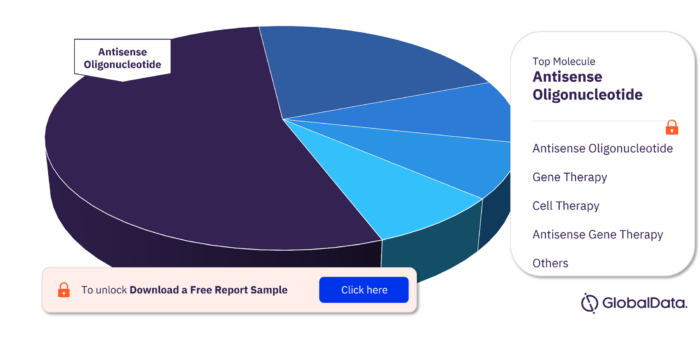
Key Molecule Types in the Dystrophin Pipeline Drugs Market
The key molecule types in the Dystrophin pipeline drugs market are Antisense Oligonucleotide, Gene Therapy, Cell Therapy, Antisense Gene Therapy, Monoclonal Antibody Conjugated, Gene-Modified Cell Therapy, Oligonucleotide, and Protein.
Dystrophin Pipeline Drugs Market, by Molecule Type, 2022 (%)
To get more insights on key molecule types, download a free sample report
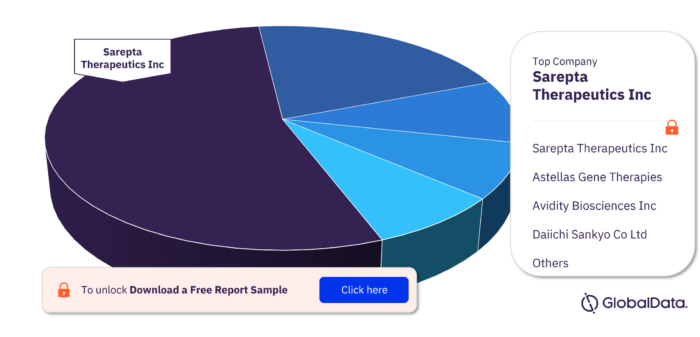
Major Companies in the Dystrophin Pipeline Drugs Market
The major companies in the Dystrophin pipeline drugs market are Sarepta Therapeutics Inc, Astellas Gene Therapies, Avidity Biosciences Inc, Daiichi Sankyo Co Ltd, Dystrogen Therapeutics SA, Editas Medicine Inc, Nippon Shinyaku Co Ltd, NS Pharma Inc, OliPass Corporation, Pepgen Ltd, Pfizer Inc, and RegenxBio Inc.
Sarepta Therapeutics Inc: Sarepta Therapeutics Inc (Sarepta) discovers and develops unique RNA-targeted medicines to treat rare diseases. The company develops its pipeline products using its multi-platform Precision Genetic Medicine Engine in gene therapy, RNA, and gene editing. The company’s platform is based on its pioneering work with phosphorodiamidate morpholino oligomer (PMO) chemistries. Its commercial products include Exondys 51, Vyondys 53 and Amondys 45 indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients who have a confirmed mutation of the DMD gene.
Dystrophin Pipeline Drugs Market, by Major Companies, 2022 (%)
For more company insights, download a free sample report
Scope
- The pipeline guide provides a snapshot of the global therapeutic landscape of Dystrophin
- The pipeline guide reviews pipeline therapeutics for Dystrophin by companies and universities/research institutes based on information derived from company and industry-specific sources.
- The pipeline guide covers pipeline products based on several stages of development ranging from pre-registration till discovery and undisclosed stages.
- The pipeline guide features descriptive drug profiles for the pipeline products which comprise, product description, descriptive licensing and collaboration details, R&D brief, MoA & other developmental activities.
- The pipeline guide reviews key companies involved in Dystrophin therapeutics and enlists all their major and minor projects.
- The pipeline guide evaluates Dystrophin therapeutics based on mechanism of action (MoA), drug target, route of administration (RoA), and molecule type.
- The pipeline guide encapsulates all the dormant and discontinued pipeline projects.
- The pipeline guide reviews the latest news related to pipeline therapeutics for Dystrophin
Reasons to Buy
- Procure strategically important competitor information, analysis, and insights to formulate effective R&D strategies.
- Recognize emerging players with a potentially strong product portfolio and create effective countercstrategies to gain a competitive advantage.
- Find and recognize significant and varied types of therapeutics under development for Dystrophin
- Classify potential new clients or partners in the target demographic.
- Develop tactical initiatives by understanding the focus areas of leading companies.
- Plan mergers and acquisitions meritoriously by identifying key players and their most promising pipeline therapeutics.
- Formulate corrective measures for pipeline projects by understanding Dystrophin pipeline depth and focus of Indication therapeutics.
- Develop and design in-licensing and out-licensing strategies by identifying prospective partners with the most attractive projects to enhance and expand business potential and scope.
- Adjust the therapeutic portfolio by recognizing discontinued projects and understand from the know-how what drove them from the pipeline.
Astellas Gene Therapies
Avidity Biosciences Inc
Code Biotherapeutics Inc
Daiichi Sankyo Co Ltd
Dystrogen Therapeutics SA
Editas Medicine Inc
Eli Lilly and Co
Evox Therapeutics Ltd
FibroGenesis LLC
MyoGene Bio LLC
Myosana Therapeutics Inc
Nippon Shinyaku Co Ltd
NS Pharma Inc
OliPass Corporation
Pepgen Ltd
Pfizer Inc
RegenxBio Inc
Sarepta Therapeutics Inc
Solid Biosciences Inc
Sutura Therapeutics Ltd
Suzhou GenAssist Therapeutics Co Ltd
Tolerion Inc
Ultragenyx Pharmaceutical Inc
Vertex Pharmaceuticals Inc
Wave Life Sciences Ltd
Table of Contents
Table
Figures
Frequently asked questions
-
What are the key therapy areas in the Dystrophin pipeline drugs market?
In the Dystrophin pipeline drugs market the key therapy areas are Genetic Disorders, and Musculoskeletal Disorders.
-
What are the key mechanisms of action in the Dystrophin pipeline drugs market?
In the Dystrophin pipeline drugs market the key mechanism of action is Dystrophin Activator.
-
What are the key routes of administration in the Dystrophin pipeline drugs market?
The key routes of administration in the Dystrophin pipeline drugs market are Intravenous, Subcutaneous, Intramuscular, and Parenteral.
-
What are the key molecule types in the Dystrophin pipeline drugs market?
The key molecule types in the Dystrophin pipeline drugs market are Antisense Oligonucleotide, Gene Therapy, Cell Therapy, Antisense Gene Therapy, Monoclonal Antibody Conjugated, Gene-Modified Cell Therapy, Oligonucleotide, and Protein.
-
What are the major companies in the Dystrophin pipeline drugs market?
In the Dystrophin pipeline drugs market the major companies are Sarepta Therapeutics Inc, Astellas Gene Therapies, Avidity Biosciences Inc, Daiichi Sankyo Co Ltd, Dystrogen Therapeutics SA, Editas Medicine Inc, Nippon Shinyaku Co Ltd, NS Pharma Inc, OliPass Corporation, Pepgen Ltd, Pfizer Inc, and RegenxBio Inc.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Other Diseases reports