Inborn Gene or Chromosome Alterations – Pipeline Products by Stage of Development 16
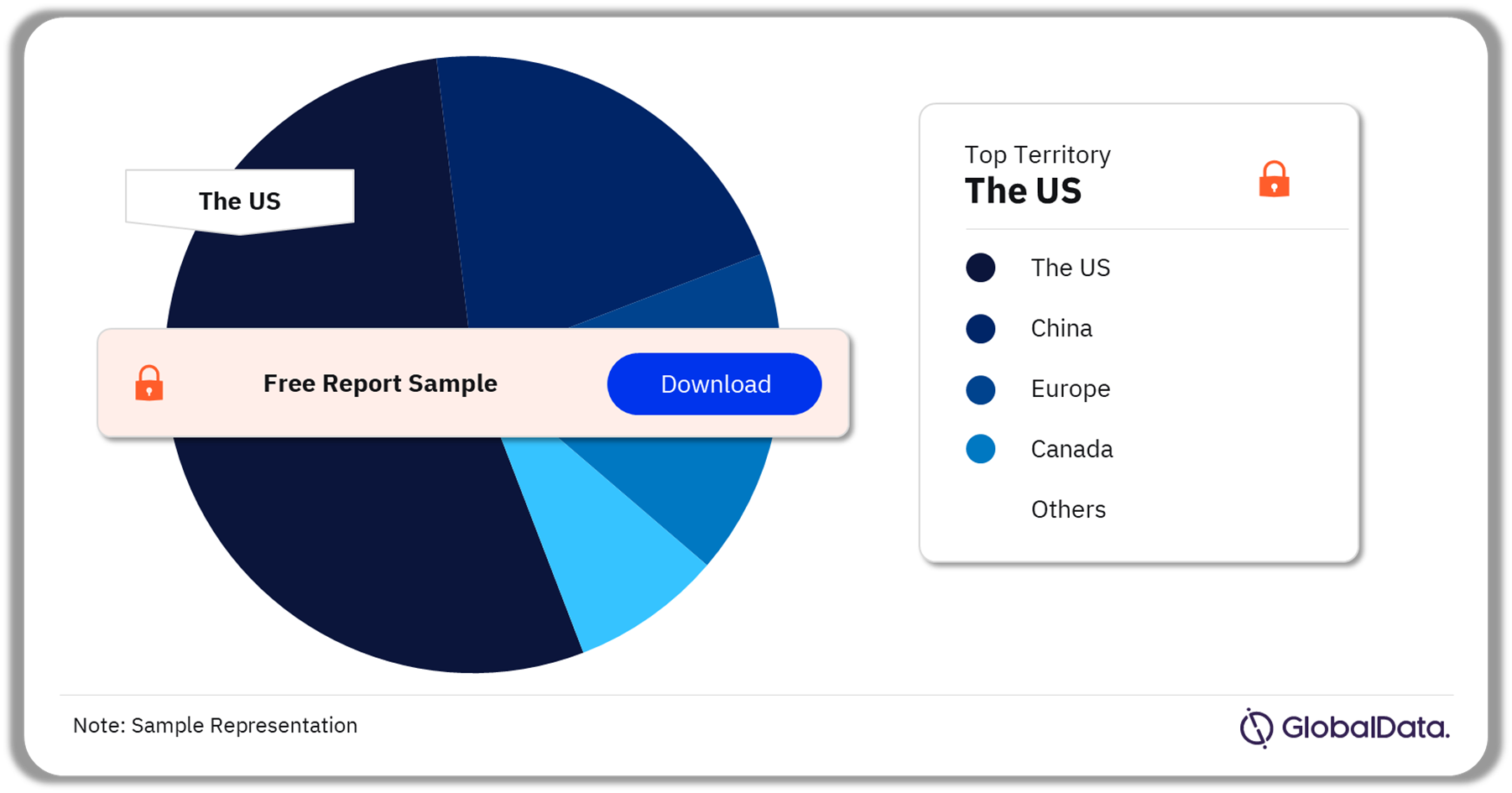
Inborn Gene or Chromosome Alterations – Pipeline Products by Territory 17
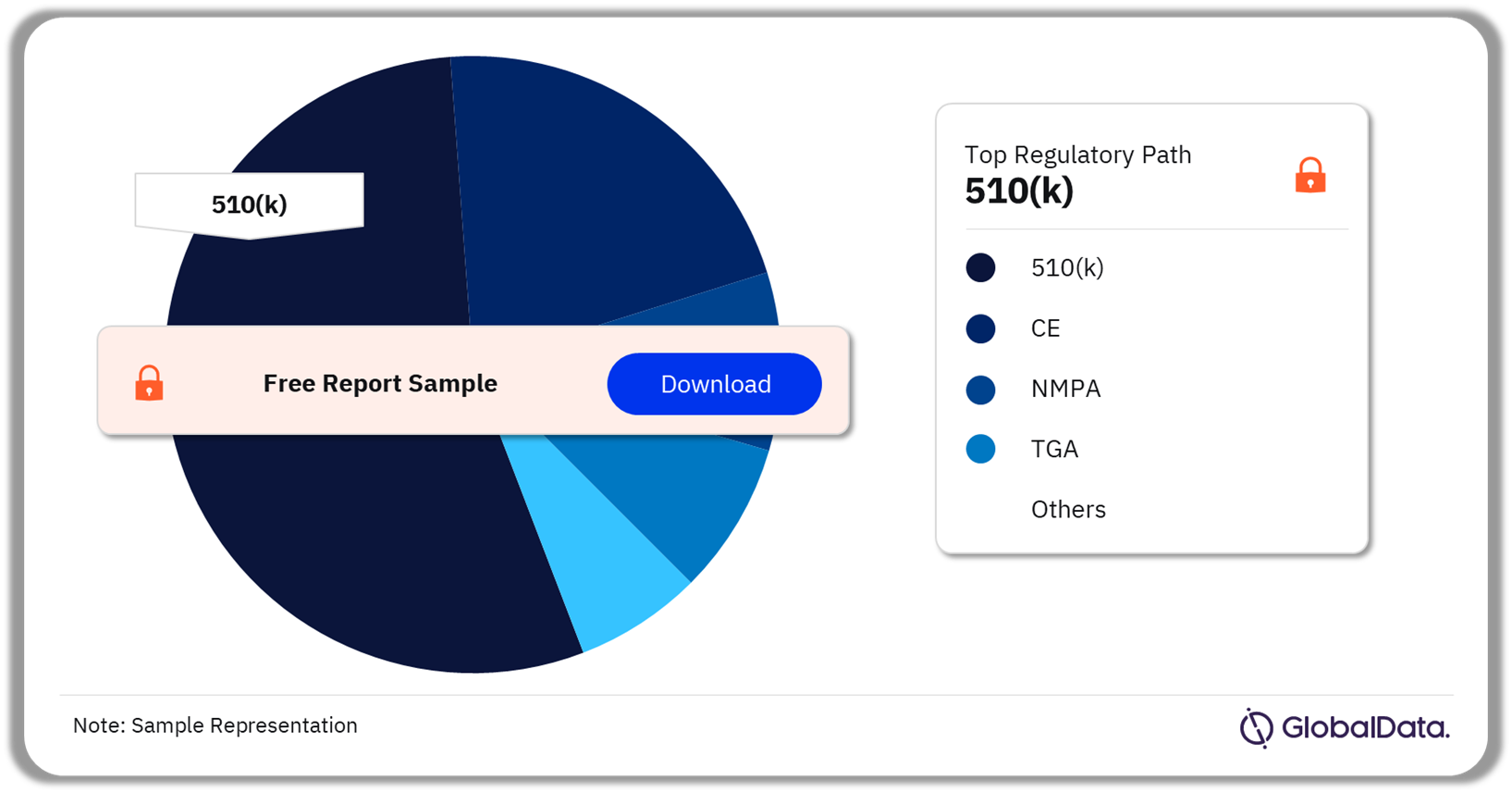
Inborn Gene or Chromosome Alterations – Pipeline Products by Regulatory Path 18
Inborn Gene or Chromosome Alterations – Pipeline Products by Estimated Approval Date 19
Inborn Gene or Chromosome Alterations – Ongoing Clinical Trials 20
Inborn Gene or Chromosome Alterations Companies – Pipeline Products by Stage of Development 21
Inborn Gene or Chromosome Alterations – Pipeline Products by Stage of Development 24
Aarhus University Pipeline Products & Ongoing Clinical Trials Overview 27
Cystic Fibrosis Urine Test – Product Status 27
Cystic Fibrosis Urine Test – Product Description 27
AC-Gen Reading Life S.L. Pipeline Products & Ongoing Clinical Trials Overview 28
Diagnostic Test – Hereditary Deafness – Product Status 28
Diagnostic Test – Hereditary Deafness – Product Description 28
Alexion Pharmaceuticals Inc Pipeline Products & Ongoing Clinical Trials Overview 29
BeginNGS – Product Status 29
BeginNGS – Product Description 29
Alexion Pharmaceuticals Inc – Ongoing Clinical Trials Overview 30
BeginNGS – Study to Assess the BeginNGS Tool for Screening Newborns for Genetic Diseases 31
Amarantus Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview 32
Diagnostic Test – IFN-Beta Resistance – Product Status 32
Diagnostic Test – IFN-Beta Resistance – Product Description 32
Arrayit Corp (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 33
PDx Pre-Symptomatic Test – Product Status 33
PDx Pre-Symptomatic Test – Product Description 33
Augusta University Pipeline Products & Ongoing Clinical Trials Overview 34
SUMO Gene Test – Type 1 Diabetes – Product Status 34
SUMO Gene Test – Type 1 Diabetes – Product Description 34
AutoGenomics Inc Pipeline Products & Ongoing Clinical Trials Overview 35
DNA Guided Diagnostic Test – Cardiovascular Disease – Product Status 35
DNA Guided Diagnostic Test – Cardiovascular Disease – Product Description 35
DNA Guided Diagnostic Test – Diabetes – Product Status 36
DNA Guided Diagnostic Test – Diabetes – Product Description 36
DNA Guided Diagnostic Test – Obesity – Product Status 36
DNA Guided Diagnostic Test – Obesity – Product Description 37
Baylor College of Medicine Pipeline Products & Ongoing Clinical Trials Overview 38
Diagnostic Assay – Rett Syndrome – Product Status 38
Diagnostic Assay – Rett Syndrome – Product Description 38
Boston Children’s Hospital Pipeline Products & Ongoing Clinical Trials Overview 39
Diagnostic Assay – Cystic Fibrosis – Product Status 39
Diagnostic Assay – Cystic Fibrosis – Product Description 39
Diagnostic Assay – Developmental Delay – Product Status 40
Diagnostic Assay – Developmental Delay – Product Description 40
CareDx Inc Pipeline Products & Ongoing Clinical Trials Overview 41
Diagnostic Test – Multiple Sclerosis – Product Status 41
Diagnostic Test – Multiple Sclerosis – Product Description 41
Diagnostic Test – Rheumatoid Arthritis – Product Status 42
Diagnostic Test – Rheumatoid Arthritis – Product Description 42
Diagnostic Test – Sjogren’s Syndrome – Product Status 42
Diagnostic Test – Sjogren’s Syndrome – Product Description 43
Diagnostic Test – Systemic Lupus Erythematosus – Product Status 43
Diagnostic Test – Systemic Lupus Erythematosus – Product Description 43
Children’s Hospital of Philadelphia Pipeline Products & Ongoing Clinical Trials Overview 44
Diagnostic Test – Cornelia De Lange Syndrome – Product Status 44
Diagnostic Test – Cornelia De Lange Syndrome – Product Description 44
Cincinnati Children’s Hospital Medical Center Pipeline Products & Ongoing Clinical Trials Overview 45
Diagnostic Biomarker Test – Biliary Atresia – Product Status 45
Diagnostic Biomarker Test – Biliary Atresia – Product Description 45
Columbia University Pipeline Products & Ongoing Clinical Trials Overview 46
Genetic Test – Schizophrenia – Product Status 46
Genetic Test – Schizophrenia – Product Description 46
Columbia University Fertility Center Pipeline Products & Ongoing Clinical Trials Overview 47
STORK Test – Product Status 47
STORK Test – Product Description 47
Daan Gene Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 48
Real Time PCR Kit – Beta-Mediterranean Anaemia – Product Status 48
Real Time PCR Kit – Beta-Mediterranean Anaemia – Product Description 48
Decipher GenX, Inc. Pipeline Products & Ongoing Clinical Trials Overview 49
Diagnostic Test – Chronic Fatigue Syndrome – Product Status 49
Diagnostic Test – Chronic Fatigue Syndrome – Product Description 49
Duke University Pipeline Products & Ongoing Clinical Trials Overview 50
Diagnostic Blood Test – Respiratory Infections – Product Status 50
Diagnostic Blood Test – Respiratory Infections – Product Description 50
Epinex Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview 51
Down Syndrome Screen Test – Product Status 51
Down Syndrome Screen Test – Product Description 51
Firalis SAS Pipeline Products & Ongoing Clinical Trials Overview 52
Prognostic miRNA Assay – Heart Failure – Product Status 52
Prognostic miRNA Assay – Heart Failure – Product Description 52
Fraunhofer Institute for Cell Therapy and Immunology Pipeline Products & Ongoing Clinical Trials Overview 53
Multimodal Early Screening Test – Dyslexia – Product Status 53
Multimodal Early Screening Test – Dyslexia – Product Description 53
Fred Hutchinson Cancer Research Center Pipeline Products & Ongoing Clinical Trials Overview 54
FANCC Gene-Based Immuno-MRM Assay – Product Status 54
FANCC Gene-Based Immuno-MRM Assay – Product Description 54
FANCI Gene-Based Immuno-MRM Assay – Product Status 55
FANCI Gene-Based Immuno-MRM Assay – Product Description 55
French National Institute of Health and Medical Research Pipeline Products & Ongoing Clinical Trials Overview 56
Biomarker Based Assay – Multiple Sclerosis – Product Status 56
Biomarker Based Assay – Multiple Sclerosis – Product Description 56
Genetic Diagnostic Kit – Bone Diseases – Product Status 57
Genetic Diagnostic Kit – Bone Diseases – Product Description 57
VLA-4 Based Assay – Duchenne Muscular Dystrophy – Product Status 57
VLA-4 Based Assay – Duchenne Muscular Dystrophy – Product Description 57
genedrive plc Pipeline Products & Ongoing Clinical Trials Overview 58
Genedrive MT-RNR1 Assay – Product Status 58
Genedrive MT-RNR1 Assay – Product Description 58
Genentech USA Inc Pipeline Products & Ongoing Clinical Trials Overview 59
Arbaclofen Companion Diagnostic Assay – Product Status 59
Arbaclofen Companion Diagnostic Assay – Product Description 59
Genomic Vision SA Pipeline Products & Ongoing Clinical Trials Overview 60
Spinal Muscular Atrophy Test – Product Status 60
Spinal Muscular Atrophy Test – Product Description 60
genomiQa Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 61
GulfDx – Product Status 61
GulfDx – Product Description 61
German Cancer Research Center Pipeline Products & Ongoing Clinical Trials Overview 62
DGKA Based Assay – Radiation-induced Fibrosis – Product Status 62
DGKA Based Assay – Radiation-induced Fibrosis – Product Description 62
German Institute of Human Nutrition Pipeline Products & Ongoing Clinical Trials Overview 63
Diagnostic Test – Obesity Associated Diabetes – Product Status 63
Diagnostic Test – Obesity Associated Diabetes – Product Description 63
Gilupi GmbH Pipeline Products & Ongoing Clinical Trials Overview 64
Diagnostic Test – Down’s Syndrome – Product Status 64
Diagnostic Test – Down’s Syndrome – Product Description 64
Helmholtz Center Munich German Research Center for Health and Environment GmbH Pipeline Products & Ongoing Clinical Trials Overview 65
2-Gene Test – Product Status 65
2-Gene Test – Product Description 65
Diagnostic Test – Pre-Diabetes – Product Status 66
Diagnostic Test – Pre-Diabetes – Product Description 66
Hummingbird Diagnostics GmbH Pipeline Products & Ongoing Clinical Trials Overview 67
Diagnostic Test – Multiple Sclerosis – Product Status 67
Diagnostic Test – Multiple Sclerosis – Product Description 67
Interleukin Genetics Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 68
Inherent Health Osteoarthritis Test – Product Status 68
Inherent Health Osteoarthritis Test – Product Description 68
Osteoporosis Risk Test – Product Status 69
Osteoporosis Risk Test – Product Description 69
Interpace Biosciences Inc Pipeline Products & Ongoing Clinical Trials Overview 70
PathFinderTG – Pancreas (Supernatant) – Product Status 70
PathFinderTG – Pancreas (Supernatant) – Product Description 70
PathFinderTG – Ulcerative Colitis Supernatant – Product Status 71
PathFinderTG – Ulcerative Colitis Supernatant – Product Description 71
Johns Hopkins University Pipeline Products & Ongoing Clinical Trials Overview 72
Biomarker Assay – Polycythemia Vera – Product Status 72
Biomarker Assay – Polycythemia Vera – Product Description 72
Diagnostic Kit – Psychiatric Disorder – Product Status 73
Diagnostic Kit – Psychiatric Disorder – Product Description 73
JS Genetics Inc Pipeline Products & Ongoing Clinical Trials Overview 74
Dyslexia Predisposition Test – Product Status 74
Dyslexia Predisposition Test – Product Description 74
LS CancerDiag Ltd Pipeline Products & Ongoing Clinical Trials Overview 75
DiagMMR – Product Status 75
DiagMMR – Product Description 75
Lucid Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview 76
EsoGuard Test – Product Status 76
EsoGuard Test – Product Description 76
Lucid Diagnostics Inc – Ongoing Clinical Trials Overview 77
EsoGuard Test – A Multicenter Case-control Study of the Efficacy of EsoGuard on Samples Collected Using EsoCheck, Versus Esophagogastroduodenoscopy, for the Diagnosis of Barrett’s Esophagus with and without Dysplasia, and for Esophageal Adenocarcinoma 78
EsoGuard Test – A Multicenter, Prospective, Open-label Registry Study to Capture Real-world Data on Esoguard Testing in at-risk Patients for the Detection of Barrett’s Esophagus or Esophageal Adenocarcinoma 78
EsoGuard Test – Clinical Utility of a Non Endoscopic Device EsoCheck and Biomarker EsoGuard as Alternative to Endoscopy for Screening for Barrett’s Esophagus in At Risk Population (ASBE) A Randomized Controlled, Virtual Patient Trial 78
EsoGuard Test – CLUE: CLinical Utility Study of EsoGuard on Samples Collected Using EsoCheck as a Triage Test for Endoscopy to Identify Barrett’s Esophagus (BE) 79
EsoGuard Test – Detection of Barrett s Esophagus in Patients Without Gastroesophageal Reflux Disease (GERD) Symptoms 79
EsoGuard Test – Genetic and Environmental Determinants of Barrett’s Esophagus and Esophageal Adenocarcinoma 79
Massachusetts Eye and Ear Infirmary Pipeline Products & Ongoing Clinical Trials Overview 80
GEDi Test – Inherited Eye Disorders – Product Status 80
GEDi Test – Inherited Eye Disorders – Product Description 80
GEDi Test – Strabismus – Product Status 81
GEDi Test – Strabismus – Product Description 81
McGill University Pipeline Products & Ongoing Clinical Trials Overview 82
Genetic Marker Test – Recurrent Abortions – Product Status 82
Genetic Marker Test – Recurrent Abortions – Product Description 82
Medical Research Council Pipeline Products & Ongoing Clinical Trials Overview 83
Epigenetic Test – Crohn’s Disease – Product Status 83
Epigenetic Test – Crohn’s Disease – Product Description 83
Genetic Test – Familial Hypercholesterolaemia – Product Status 84
Genetic Test – Familial Hypercholesterolaemia – Product Description 84
Molecular Stethoscope Inc Pipeline Products & Ongoing Clinical Trials Overview 85
Next-Generation cf-mRNA Test – Alzheimer’s Disease – Product Status 85
Next-Generation cf-mRNA Test – Alzheimer’s Disease – Product Description 85
Nanosphere Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 86
Verigene Processor SP System – Hemochromatosis (HFE) Nucleic Acid Test – Product Status 86
Verigene Processor SP System – Hemochromatosis (HFE) Nucleic Acid Test – Product Description 86
National University of Singapore Pipeline Products & Ongoing Clinical Trials Overview 87
Rapid Screening Assay – Fragile X Syndrome – Product Status 87
Rapid Screening Assay – Fragile X Syndrome – Product Description 87
Nel ASA Pipeline Products & Ongoing Clinical Trials Overview 88
AMYtect – Product Status 88
AMYtect – Product Description 88
NewGene Ltd Pipeline Products & Ongoing Clinical Trials Overview 89
Limb Girdle Muscular Dystrophy Test – Product Status 89
Limb Girdle Muscular Dystrophy Test – Product Description 89
Oxford Biodynamics Plc Pipeline Products & Ongoing Clinical Trials Overview 90
Companion Diagnostic Test – Amyotrophic Lateral Sclerosis – Product Status 90
Companion Diagnostic Test – Amyotrophic Lateral Sclerosis – Product Description 91
Companion Diagnostic Test – Corticobasal Degeneration – Product Status 91
Companion Diagnostic Test – Corticobasal Degeneration – Product Description 91
Companion Diagnostic Test – Dementia With Lewy Bodies – Product Status 92
Companion Diagnostic Test – Dementia With Lewy Bodies – Product Description 92
Companion Diagnostic Test – Diffuse Lewy Body Disease – Product Status 92
Companion Diagnostic Test – Diffuse Lewy Body Disease – Product Description 93
Companion Diagnostic Test – Frontotemporal Lobar Degeneration – Product Status 93
Companion Diagnostic Test – Frontotemporal Lobar Degeneration – Product Description 93
Companion Diagnostic Test – Multiple System Atrophy – Product Status 94
Companion Diagnostic Test – Multiple System Atrophy – Product Description 94
Companion Diagnostic Test – Osteoporosis – Product Status 94
Companion Diagnostic Test – Osteoporosis – Product Description 95
Companion Diagnostic Test – Parkinsonism-Dementia-Amyotrophic Lateral Sclerosis Complex – Product Status 95
Companion Diagnostic Test – Parkinsonism-Dementia-Amyotrophic Lateral Sclerosis Complex – Product Description 95
Companion Diagnostic Test – Progressive Supranuclear Palsy – Product Status 96
Companion Diagnostic Test – Progressive Supranuclear Palsy – Product Description 96
Companion Diagnostic Test – Sarcopenia – Product Status 96
Companion Diagnostic Test – Sarcopenia – Product Description 97
EpiSwitch Parkinson’s Disease Diagnostic Test – Product Status 97
EpiSwitch Parkinson’s Disease Diagnostic Test – Product Description 97
Population Bio Inc Pipeline Products & Ongoing Clinical Trials Overview 98
Genetic Test – Amyotrophic Lateral Sclerosis – Product Status 98
Genetic Test – Amyotrophic Lateral Sclerosis – Product Description 99
Genetic Test – Bipolar Disorder – Product Status 99
Genetic Test – Bipolar Disorder – Product Description 99
Genetic Test – Diabetes Mellitus – Product Status 100
Genetic Test – Diabetes Mellitus – Product Description 100
Genetic Test – Epilepsy – Product Status 100
Genetic Test – Epilepsy – Product Description 101
Genetic Test – Multiple Sclerosis – Product Status 101
Genetic Test – Multiple Sclerosis – Product Description 101
Genetic Test – Obesity – Product Status 102
Genetic Test – Obesity – Product Description 102
Genetic Test – Progressive Multifocal Leukoencephalopathy – Product Status 102
Genetic Test – Progressive Multifocal Leukoencephalopathy – Product Description 103
Genetic Test – Pulmonary Fibrosis – Product Status 103
Genetic Test – Pulmonary Fibrosis – Product Description 103
Genetic Test – Rheumatoid Arthritis – Product Status 104
Genetic Test – Rheumatoid Arthritis – Product Description 104
Genetic Test – Schizophrenia – Product Status 104
Genetic Test – Schizophrenia – Product Description 105
Precipio Inc Pipeline Products & Ongoing Clinical Trials Overview 106
Diagnostic Test – Parkinson’s Disease – Product Status 106
Diagnostic Test – Parkinson’s Disease – Product Description 106
Predigen Inc Pipeline Products & Ongoing Clinical Trials Overview 107
Predigen Autoimmune/Inflammatory Disease Test – Product Status 107
Predigen Autoimmune/Inflammatory Disease Test – Product Description 107
Quest Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview 108
Genotyping Assay – Cystic Fibrosis – Product Status 108
Genotyping Assay – Cystic Fibrosis – Product Description 108
Genotyping Assay – Fragile X Syndrome – Product Status 109
Genotyping Assay – Fragile X Syndrome – Product Description 109
Revvity Inc Pipeline Products & Ongoing Clinical Trials Overview 110
PerkinElmer Five-Plex qPCR Assay – Product Status 110
PerkinElmer Five-Plex qPCR Assay – Product Description 110
Sapien Biosciences Pipeline Products & Ongoing Clinical Trials Overview 111
Genetic Testing Panel – Autism – Product Status 111
Genetic Testing Panel – Autism – Product Description 111
Genetic Testing Panel – Diabetes – Product Status 112
Genetic Testing Panel – Diabetes – Product Description 112
Genetic Testing Panel – Perinatal Disease – Product Status 112
Genetic Testing Panel – Perinatal Disease – Product Description 112
Scipher Medicine Corp Pipeline Products & Ongoing Clinical Trials Overview 113
PrismUC – Product Status 113
PrismUC – Product Description 113
Seattle Children’s Hospital Pipeline Products & Ongoing Clinical Trials Overview 114
Diagnostic Test – Congenital Disorders – Product Status 114
Diagnostic Test – Congenital Disorders – Product Description 114
Seegene Inc Pipeline Products & Ongoing Clinical Trials Overview 115
Allplex – Thrombosis Assay – Product Status 115
Allplex – Thrombosis Assay – Product Description 115
Sophia Genetics SA Pipeline Products & Ongoing Clinical Trials Overview 116
NGS Test – Cardiac Disease – Product Status 116
NGS Test – Cardiac Disease – Product Description 116
Strand Life Sciences Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 117
Next-generation Sequencing Based Test – Hereditary Epilepsy – Product Status 117
Next-generation Sequencing Based Test – Hereditary Epilepsy – Product Description 117
Next-generation Sequencing Based Test – Hereditary PI – Product Status 118
Next-generation Sequencing Based Test – Hereditary PI – Product Description 118
SUNY Upstate Medical University Pipeline Products & Ongoing Clinical Trials Overview 119
Diagnostic Assay – Inflammatory Bowel Disease Risk – Product Status 119
Diagnostic Assay – Inflammatory Bowel Disease Risk – Product Description 119
Suzhou Basecare Medical Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 120
PGT-SR Test Kit – Product Status 120
PGT-SR Test Kit – Product Description 120
WES Test Kit – Product Status 121
WES Test Kit – Product Description 121
TBG Diagnostics Ltd Pipeline Products & Ongoing Clinical Trials Overview 122
TBG Kit – Hereditary Genetic Disease – Product Status 122
TBG Kit – Hereditary Genetic Disease – Product Description 122
Tel Aviv University Pipeline Products & Ongoing Clinical Trials Overview 123
Blood Test – Genetic Disorders – Product Status 123
Blood Test – Genetic Disorders – Product Description 123
Transplant Genomics Inc Pipeline Products & Ongoing Clinical Trials Overview 124
Diagnostic Test – Heart Transplantation – Product Status 124
Diagnostic Test – Heart Transplantation – Product Description 124
University of California San Diego Pipeline Products & Ongoing Clinical Trials Overview 125
Magnetic Nanosensor-Based miRNA Assay – Product Status 125
Magnetic Nanosensor-Based miRNA Assay – Product Description 125
University of Central Florida Pipeline Products & Ongoing Clinical Trials Overview 126
hMRS Based Genomic Assay – Mycobacterium – Product Status 126
hMRS Based Genomic Assay – Mycobacterium – Product Description 126
PCR-Free Nucleic Acid Sequences Assay – Product Status 127
PCR-Free Nucleic Acid Sequences Assay – Product Description 127
University of Colorado Pipeline Products & Ongoing Clinical Trials Overview 128
CBS Gene Mutation Test – Product Status 128
CBS Gene Mutation Test – Product Description 128
University of Illinois at Chicago Pipeline Products & Ongoing Clinical Trials Overview 129
Diagnostic Test – Complicated Sarcoidosis – Product Status 129
Diagnostic Test – Complicated Sarcoidosis – Product Description 129
University of Michigan Pipeline Products & Ongoing Clinical Trials Overview 130
Diagnostic Test – BRAF Negative Langerhans Cell Histiocytosis – Product Status 130
Diagnostic Test – BRAF Negative Langerhans Cell Histiocytosis – Product Description 130
Genetic Test – Neuron Disease – Product Status 131
Genetic Test – Neuron Disease – Product Description 131
University of Pennsylvania Pipeline Products & Ongoing Clinical Trials Overview 132
Diagnostic Test – Upper Respiratory Tract Infection – Product Status 132
Diagnostic Test – Upper Respiratory Tract Infection – Product Description 132
University of Pittsburgh Pipeline Products & Ongoing Clinical Trials Overview 133
AuguryDx – Aneuploidy Assay – Product Status 133
AuguryDx – Aneuploidy Assay – Product Description 133
Biomarker Assay – Fetal Genetic Disease – Product Status 134
Biomarker Assay – Fetal Genetic Disease – Product Description 134
Vanderbilt University Pipeline Products & Ongoing Clinical Trials Overview 135
Diagnostic Assay – Premature Birth – Product Status 135
Diagnostic Assay – Premature Birth – Product Description 135
Vanessa Biotech Pipeline Products & Ongoing Clinical Trials Overview 136
Microvillus Inclusion Disease Assay – Product Status 136
Microvillus Inclusion Disease Assay – Product Description 136
Virginia Commonwealth University Pipeline Products & Ongoing Clinical Trials Overview 137
Gene Based Test – Risk of Premature Birth – Product Status 137
Gene Based Test – Risk of Premature Birth – Product Description 137
VitaPath Genetics, Inc. Pipeline Products & Ongoing Clinical Trials Overview 138
Diagnostic Test – Spina Bifida – Product Status 138
Diagnostic Test – Spina Bifida – Product Description 138
Glossary 203
![]()