Breast Implants Pipeline by Development Stages, Segments, Region and Countries, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Breast Implants Pipeline Market Report Overview
Breast Implants are also known as intensive-care ventilators. They provide artificial respiratory support to critical patients in ICUs. The Breast Implants pipeline market research report provides a comprehensive understanding of the pipeline products along with their comparative analysis at various stages of development. The report also includes information about the territories wherein the clinical trials are in progress, the regulatory paths followed by the trials, and the companies associated with the trials.
| Key Segments | · Implants
· Shaped Gel Implants · Round Gel Implants · Breast Meshes |
| Key Territories | · The US
· Europe · China · Australia · South Korea |
| Key Regulatory Paths | · PMA
· CE · NMPA · 510(k) · TGA |
| Leading Companies | · 3D Bio Corp
· Allergan Aesthetics · Apex Medical Device Design LLC · Arula Technologies · BellaSeno GmbH |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Breast Implants Pipeline Market Segments
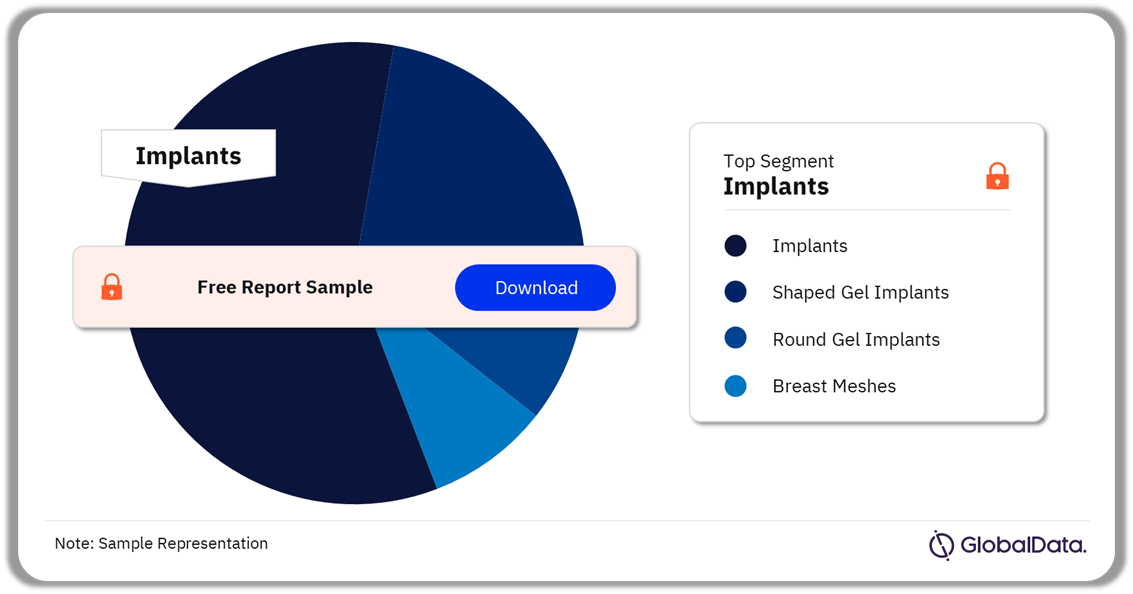
The Breast Implants pipeline market can be categorized into implants, shaped gel implants, round, gel implants, and breast meshes. As of March 2024, the implants segment accounted for the highest share of the Breast Implants pipeline market.
Breast Implants Pipeline Market Analysis by Segments, 2024 (%)
Buy the Full Report for More Insights into the Breast Implants Pipeline Market Segments
Pipeline Market Segmentation by Territories
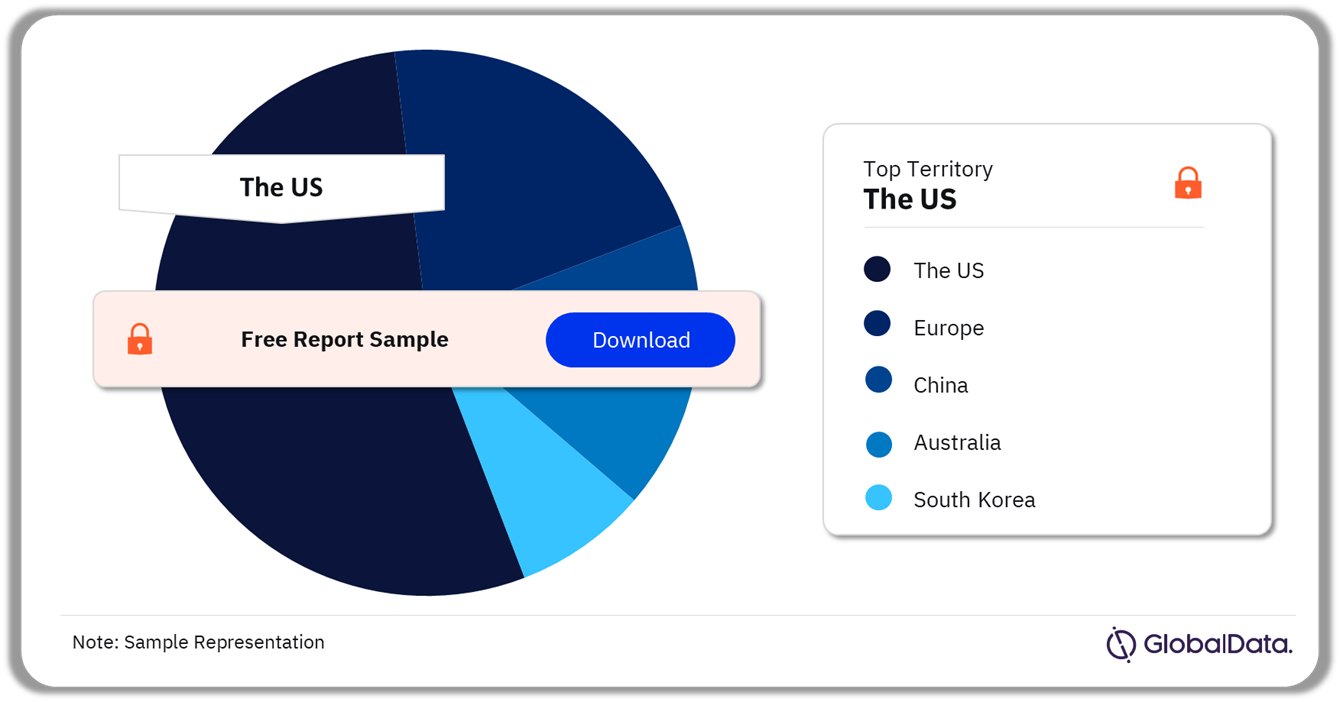
A few of the key territories for the Breast Implants pipeline market are the US, Europe, China, Australia, and South Korea among others. As of March 2024, the US accounted for the highest number of pipeline products.
Breast Implants Pipeline Market Analysis by Territories, 2024 (%)
Buy the Full Report for More Territory Insights into the Breast Implants Pipeline Market
Breast Implants Pipeline Market Segmentation by Regulatory Paths
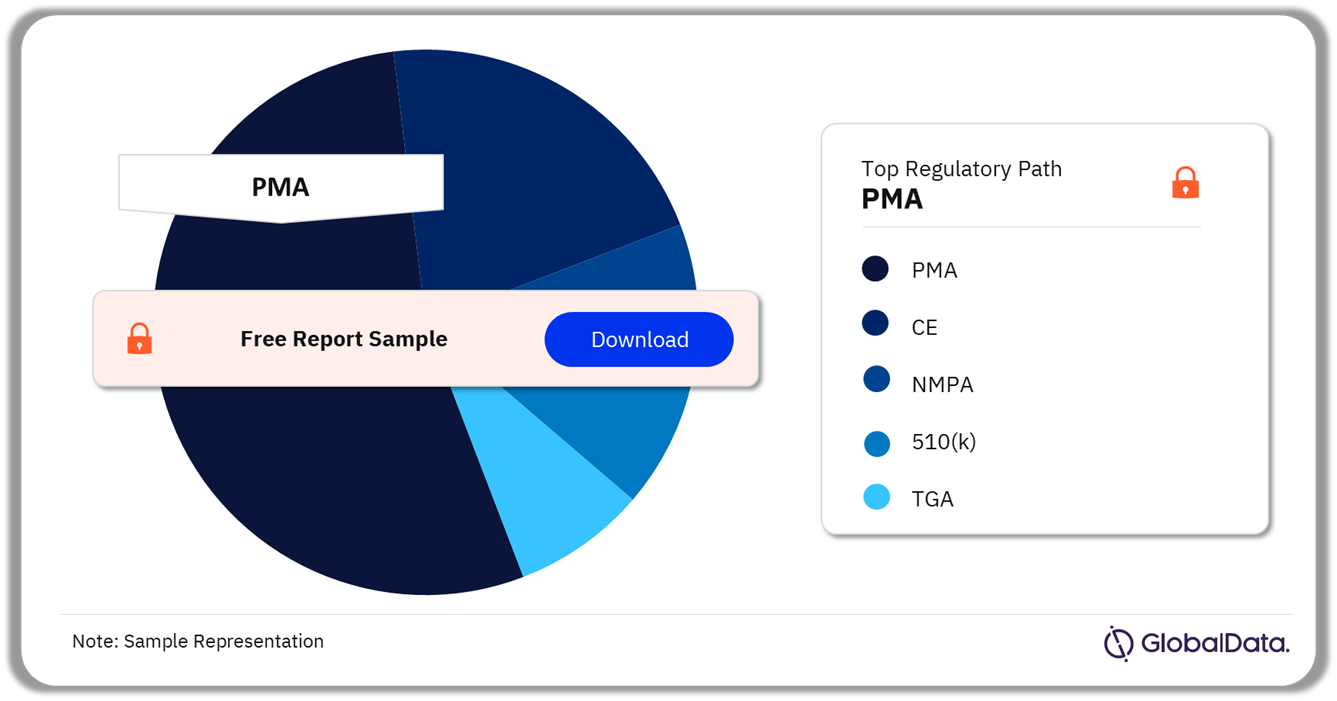
The key regulatory paths in the Breast Implants pipeline products market are PMA, CE, NMPA, 510(k), and TGA as of March 2024, PMA was the most followed pathway for pipeline products in the market.
Breast Implants Pipeline Market Analysis by Regulatory Paths, 2024 (%)
Buy the Full Report for More Regulatory Path Insights into the Breast Implants Pipeline Market
Breast Implants Pipeline Market – Competitive Landscape
A few of the key companies associated with the Breast Implants pipeline market are 3D Bio Corp, Allergan Aesthetics, Apex Medical Device Design LLC, Arula Technologies, and BellaSeno GmbH among others.
3D Bio Corp (3D Bio): 3D Bio Corp is a biologic and bioprinting company. The company operates as a biotechnology company that specializes in regenerative medicine and devices. The company’s pipeline products include AuriNovo, AnnuNovo, DiscNovo, and NasaNovo. It offers a bioprinting platform with an emphasis on living implants made of whole tissue for immediate use. The company also has lab and factory facilities in Ithaca, New York, and an engineering workshop in Brooklyn, New York. The company received funding from Kairos Ventures. 3D Bio is headquartered in New York, the US.
Allergan Aesthetics: Allergan Aesthetics is a pharmaceutical company that develops, manufactures, and markets a portfolio of leading aesthetics brands and products. It is headquartered in Irvine, California the US.
Breast Implants Pipeline Market Analysis by Companies, 2024
Buy the Full Report for More Company Insights into the Breast Implants Pipeline Market
Segments Covered in the Report
Breast Implants Pipeline Market Territories Outlook
- The US
- Europe
- China
- Australia
- South Korea
Breast Implants Pipeline Market Regulatory Paths Outlook
- PMA
- CE
- NMPA
- 510(k)
- TGA
Scope
This report provides:
- Extensive coverage of the Breast Implants under development.
- Exhaustive list of major pipeline products and their details, including product description, licensing and collaboration details, and other developmental activities.
- Reviews of major players involved in the development of Breast Implants and lists all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from early development to approved/issued stage.
- Key clinical trial data of ongoing clinical trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with potentially strong product portfolios and create effective counter strategies to gain a competitive advantage.
- Identify and understand important and diverse types of Breast Implants under development.
- Develop market-entry and market-expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- Carry out an in-depth analysis of the product’s current stage of development, territory, and estimated launch date.
Allergan Aesthetics
Apex Medical Device Design LLC
Arula Technolgies
BellaSeno GmbH
BioPlus Co Ltd
Biostruxs LLC
Collplant Biotechnologies Ltd
Establishment Labs SA
FixNip Ltd
GC Aesthetics Plc
Healshape SAS
ImpLite Ltd.
Innovia LLC
Lattice Medical
LifeCell Corp
Melodi Health Inc
Mentor Worldwide LLC
MTF Biologics
Neosthetic, LLC
Osteopore International Pte Ltd
Plcoskin Co Ltd
PolyNovo Biomaterials Pty Ltd
Prayasta 3D Inventions Pvt Ltd
Surgalign Spine Technologies Inc
Symatese Aesthetics
Tensive SRL
TeVido BioDevices LLC
Table of Contents
Table
Figures
Frequently asked questions
-
Which territory had the highest number of products in the Breast Implants pipeline market?
As of March 2024, the US accounted for the highest number of pipeline products in the Breast Implants market.
-
Which was the leading segment in the Breast Implants pipeline market?
As of March 2024, Implants was the leading segment in the Breast Implants pipeline market.
-
Which was the most followed regulatory pathway in the Breast Implants pipeline market?
As of March 2024, PMA was the most followed pathway for pipeline products in the Breast Implants market.
-
Which are the major companies operating in the Breast Implants pipeline market?
A few of the key companies associated with the Breast Implants pipeline market are 3D Bio Corp, Allergan Aesthetics, Apex Medical Device Design LLC, Arula Technologies, and BellaSeno GmbH among others.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Breast Implants reports