Negative Pressure Wound Therapy (NPWT) Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Negative Pressure Wound Therapy (NPWT) Pipeline Market Report Overview
Negative Pressure Wound Therapy (NPWT) is used to treat acute and chronic wounds. A vacuum source of an NPWT device creates continuous or intermittent negative pressure inside the wound to remove fluid, exudates, and infectious materials which helps in healing and closure. The Negative Pressure Wound Therapy (NPWT) pipeline market research report provides comprehensive information about the NPWT pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials that are in progress.
| Key Segments | · NPWT Devices
· Disposable NPWT Devices · NPWT Devices & Accessories · NPWT Dressings · Portable NPWT Devices |
| Key Territories | · The US
· Europe · India · New Zealand |
| Key Regulatory Paths | · 510(k)
· CE · ICAC · TGA · de novo |
| Leading Companies | · Aatru Medical LLC
· Acelity L.P. Inc. · Adept Medical Ltd · Aroa Biosurgery Ltd · Ceramatec Inc · CloSure Inc · ConvaTec Group Plc · DeRoyal Industries Inc · Healyx Labs Inc · Invacare Corporation |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
NPWT Pipeline Market by Segments
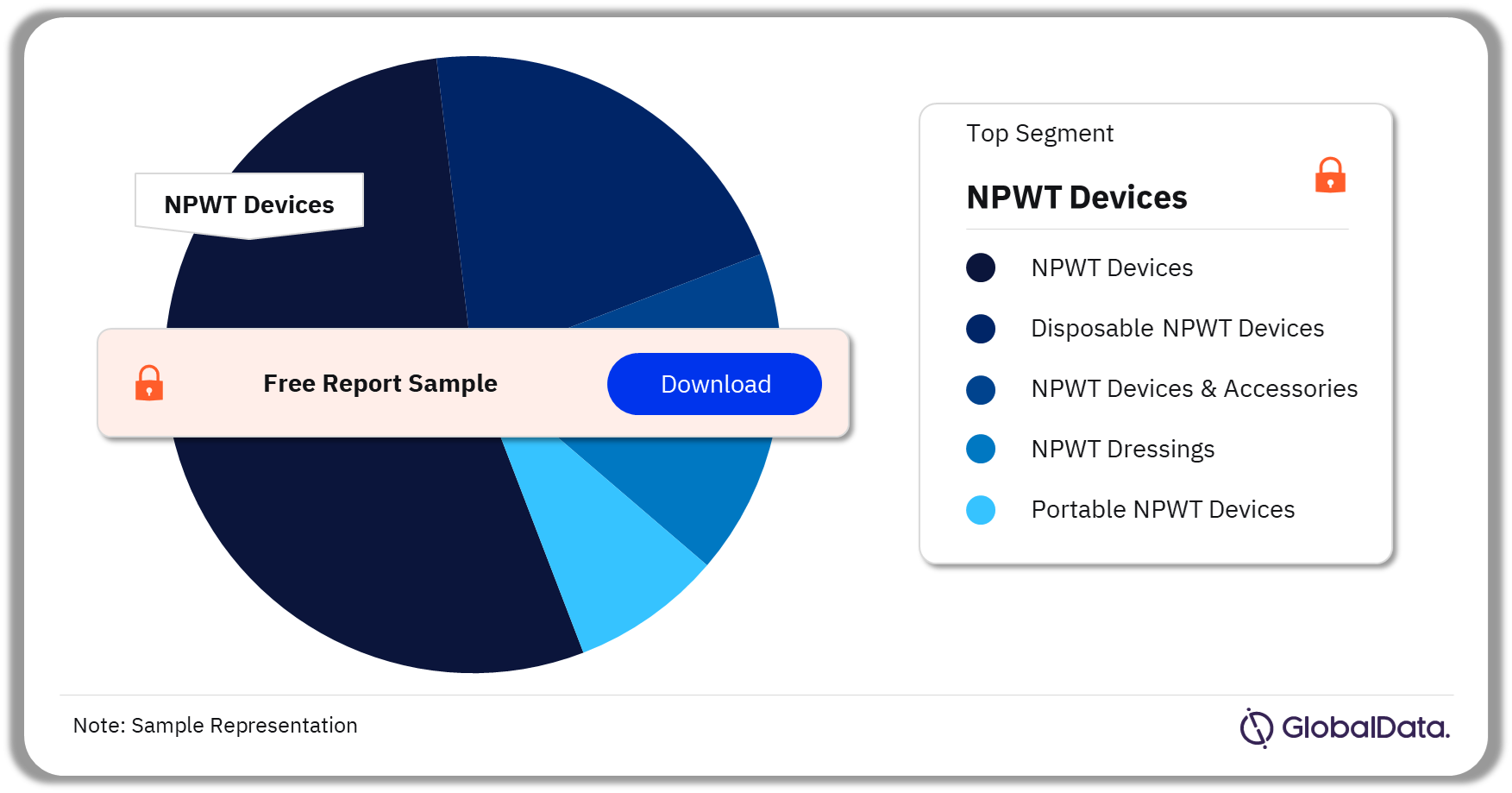
The key segments in the NPWT pipeline market are NPWT devices, disposable NPWT devices, NPWT devices and accessories, NPWT dressings, and portable NPWT devices. As of September 2023, NPWT devices have the highest number of pipeline products.
NPWT Pipeline Market Analysis by Segments, 2023 (%)
Buy Full Report for More Segment Insights into the NPWT Pipeline Market
NPWT Pipeline Market Segmentation by Territories
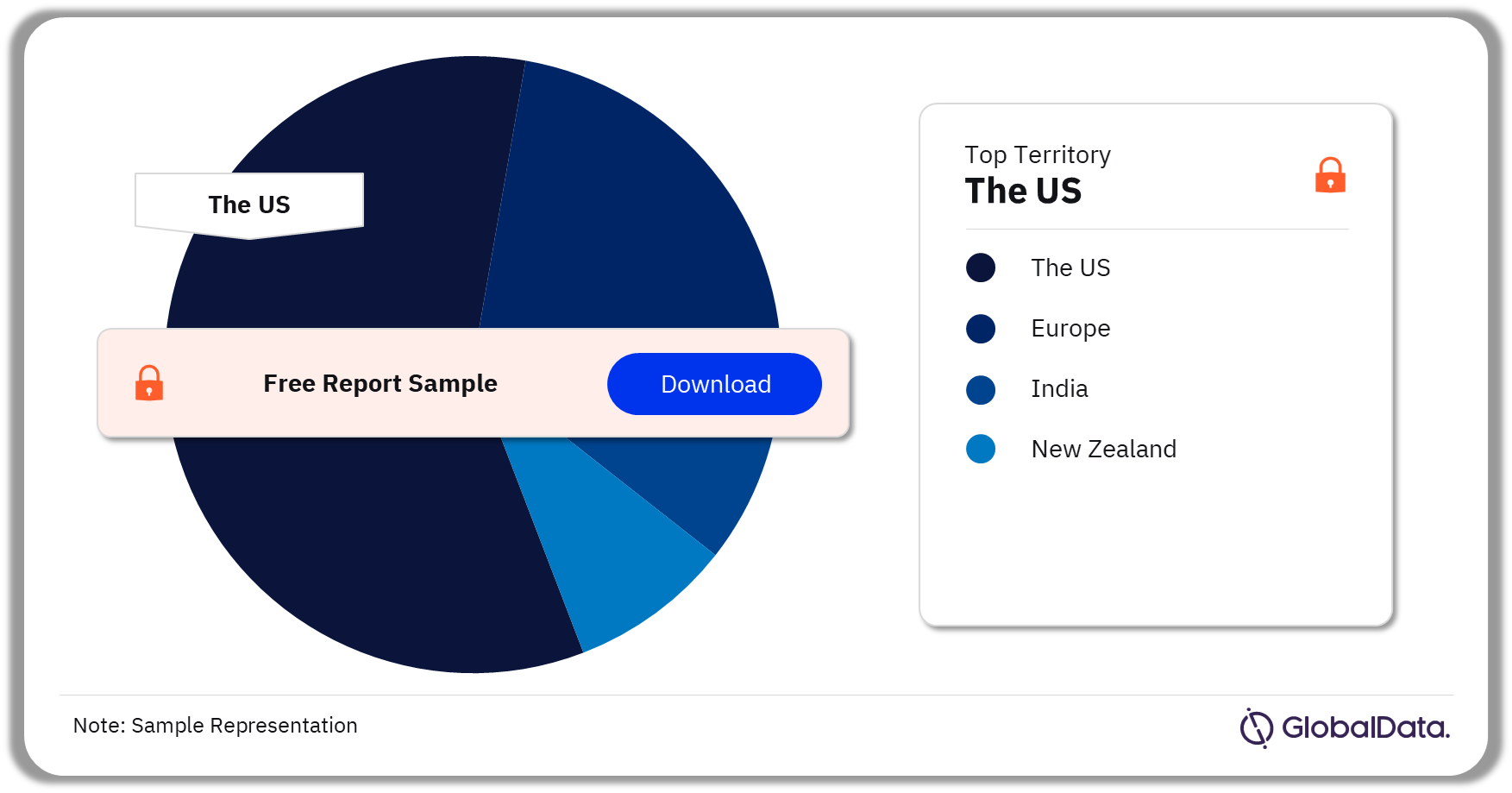
Some of the key territories in the NPWT market are the US, Europe, India, and New Zealand. As of September 2023, the US has the highest number of pipeline products.
NPWT Pipeline Market Analysis by Territories, 2023 (%)
Buy Full Report for More Territory Insights into the NPWT Pipeline Market
NPWT Pipeline Market Segmentation by Regulatory Paths
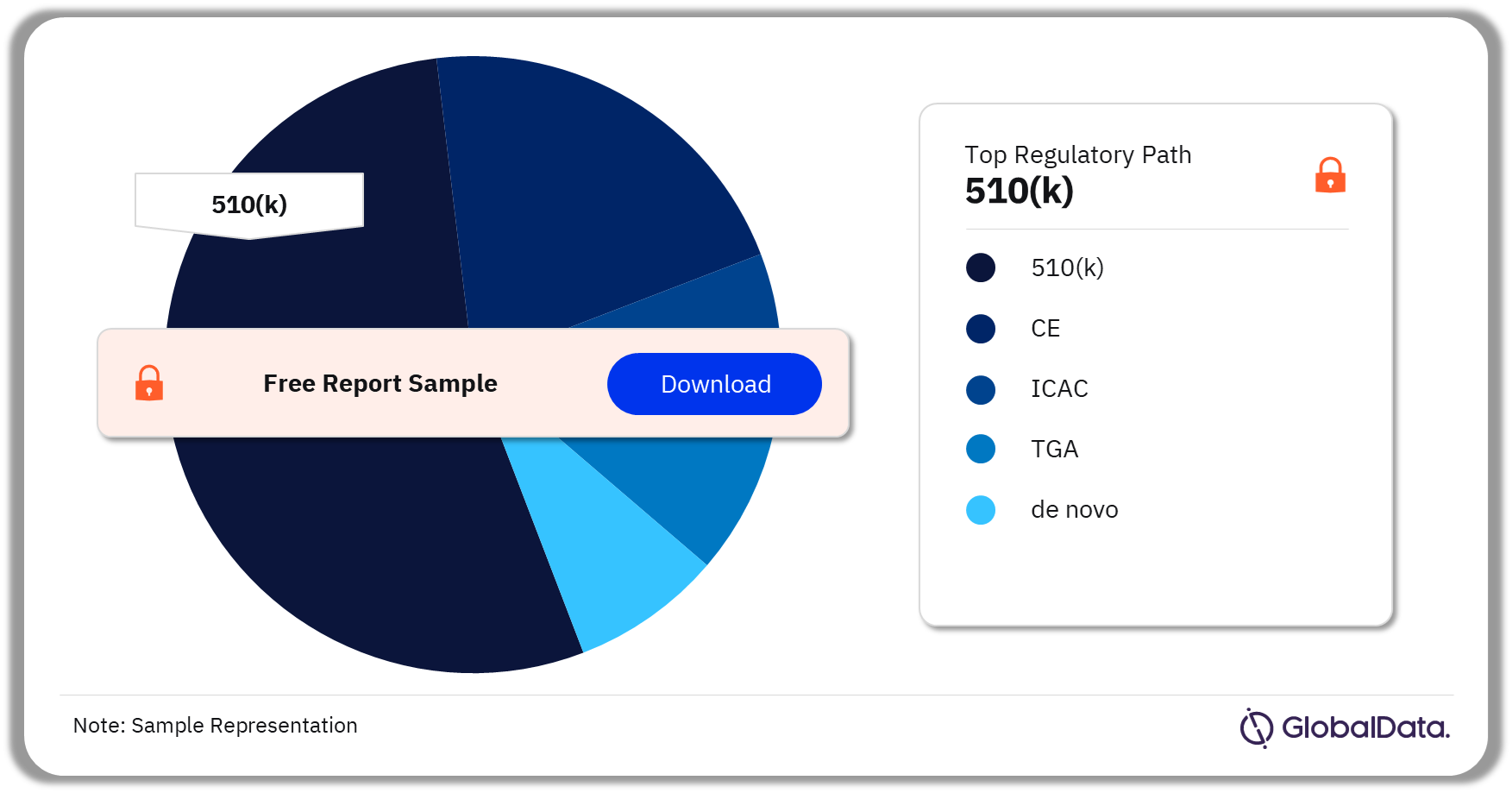
Some of the key regulatory paths in the NPWT pipeline market are 510(k), CE, ICAC, TGA, and de novo. As of September 2023, 510(k) is the most followed pathway for pipeline products in the market.
NPWT Pipeline Market Analysis by Regulatory Paths, 2023 (%)
Buy Full Report for More Regulatory Path Insights into the NPWT Pipeline Market
NPWT Pipeline Market – Competitive Landscape
Some of the key companies in the NPWT pipeline market are Aatru Medical LLC, Acelity L.P. Inc., Adept Medical Ltd, Aroa Biosurgery Ltd, Ceramatec Inc, CloSure Inc, ConvaTec Group Plc, DeRoyal Industries Inc, Healyx Labs Inc, and Invacare Corporation.
Aatru Medical LLC: It is a medical device company that specializes in technology development in negative pressure wound therapy for surgical incisions and chronic wounds. Aatru is headquartered in Cleveland, Ohio, the US.
Acelity L.P. Inc.: It is an advanced wound care company that, through its subsidiaries, develops and commercializes medical devices in the areas of wound management, plastic and reconstructive surgery, incision management, epidermal harvesting, and animal health. The company’s operations are spread across North America, Australasia, Europe, the Americas and East Asia. Acelity is headquartered in San Antonio, Texas, the US.
NPWT Pipeline Market Analysis by Companies, 2023
Buy Full Report for More Company Insights into the NPWT Pipeline Market
Segments Covered in the Report
NPWT Pipeline Market Segments Outlook
- NPWT Devices
- Disposable NPWT Devices
- NPWT Devices & Accessories
- NPWT Dressings
- Portable NPWT Devices
NPWT Pipeline Market Territories Outlook
- The US
- Europe
- India
- New Zealand
NPWT Pipeline Market Regulatory Paths Outlook
- 510(k)
- CE
- ICAC
- TGA
- de novo
Scope
This report provides:
- Extensive coverage of the Negative Pressure Wound Therapy (NPWT) under development.
- Review details of major pipeline products which include product description, licensing and collaboration details and other developmental activities including pipeline territories, regulatory paths, and estimated approval dates.
- Reviews of major players involved in the pipeline product development.
- Provides key clinical trial data related to ongoing clinical trials such as trial phase, trial status, trial start and end dates, and the number of trials of the major Negative Pressure Wound Therapy (NPWT) pipeline products.
- Review of recent developments in the segment/industry.
Reasons to Buy
This report provides:
- Extensive coverage of the Negative Pressure Wound Therapy (NPWT) under development.
- Review details of major pipeline products which include product description, licensing and collaboration details and other developmental activities including pipeline territories, regulatory paths, and estimated approval dates.
- Reviews of major players involved in the pipeline product development.
- Provides key clinical trial data related to ongoing clinical trials such as trial phase, trial status, trial start and end dates, and the number of trials of the major Negative Pressure Wound Therapy (NPWT) pipeline products.
- Review of recent developments in the segment/industry.
Acelity L.P. Inc.
Adept Medical Ltd
Aroa Biosurgery Ltd
Ceramatec Inc
CloSure Inc
ConvaTec Group Plc
DeRoyal Industries Inc
Healyx Labs Inc
Invacare Corporation
Ion-Vac Inc
MEDICUD Srl
MLM Biologics Inc
Montana State University
Nexgel Inc
Otivio AS
Progenerative Medical Inc
ReHeal LLC
Rmmedi Innovations Pvt Ltd
S2Medical AB
Shieldheart Medtech AB
Smith & Nephew Medical Ltd
Smith & Nephew Plc
UMass Chan Medical School
University of Pisa
Wake Forest Baptist Medical Center
Worldwide Innovative Healthcare, Inc.
Table of Contents
Table
Figures
Frequently asked questions
-
Which is the leading segment in the NPWT pipeline market?
NPWT devices is the leading segment in the NPWT pipeline market as of September 2023.
-
Which territory has the highest number of pipeline products in the NPWT pipeline market?
As of September 2023, the US has the highest number of products in the NPWT pipeline market.
-
Which is the most followed regulatory pathway in the NPWT pipeline market?
As of September 2023, 510(k) is the most followed pathway for products in the NPWT pipeline market.
-
Who are the major companies operating in the NPWT pipeline market?
Some of the key companies in the NPWT pipeline market are Aatru Medical LLC, Acelity L.P. Inc., Adept Medical Ltd, Aroa Biosurgery Ltd, Ceramatec Inc, CloSure Inc, ConvaTec Group Plc, DeRoyal Industries Inc, Healyx Labs Inc, and Invacare Corporation.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Negative Pressure Wound Therapy (NPWT) reports