Prosthetic Heart Valves Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Prosthetic Heart Valves Pipeline Market Report Overview
Prosthetic heart valves is a device which mimics the function of human heart valves and have a passive mode of functioning; opening and closing of valves are responses to pressure and flow changes within the heart. They are for the patients that have a heart valvular disease where they need their valve to be replaced.
The Prosthetic Heart Valves pipeline market research report provides comprehensive information about the Prosthetic Heart Valves pipeline products with comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
Prosthetic Heart Valves Pipeline Market Segments
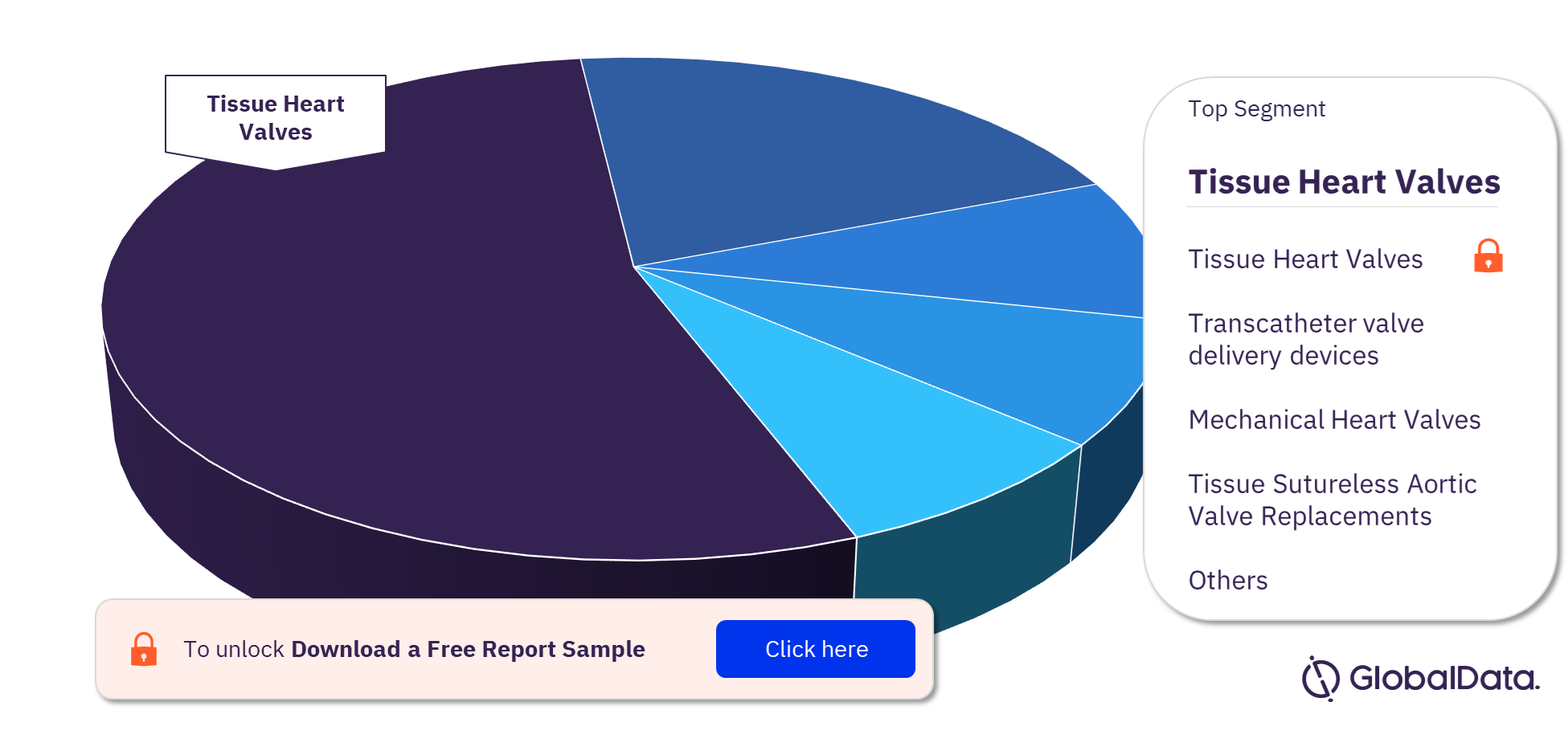
The key segments in the Prosthetic Heart Valves pipeline market are Tissue Heart Valves, Transcatheter valve delivery devices, Mechanical Heart Valves, Tissue Sutureless Aortic Valve Replacements, Tissue Sutured Mitral Valve Replacements, Mechanical Sutured Mitral Valve Replacements, and Tissue Sutured Aortic Valve Replacements. As of April 2023, Tissue Heart Valves has the highest number of pipeline products.
Tissue Heart Valves: They are a combination of tissue and synthetic biomaterials with the tissue itself being flexible. Bovine and porcine valves are included here. This segment comprises of Tissue Sutured Mitral Valve Replacements, Tissue Sutureless Aortic Valve Replacements and Tissue Sutured Aortic Valve Replacements.
Prosthetic Heart Valves Pipeline Market Analysis, by Segments, 2023 (%)
For more segment insights into the Prosthetic Heart Valves pipeline market, download a free report sample
Prosthetic Heart Valves Pipeline Market Segmentation by Territories
Some of the key territories in the Prosthetic Heart Valves pipeline market are the US, Europe, China, India, Israel, Japan, Canada, Singapore, South Korea, and Taiwan. As of April 2023, the US has the highest number of pipeline products.
Prosthetic Heart Valves Pipeline Market Analysis, by Territories, 2023 (%)
For more territory insights into the Prosthetic Heart Valves pipeline market, download a free report sample
Prosthetic Heart Valves Pipeline Market Segmentation by Regulatory Paths
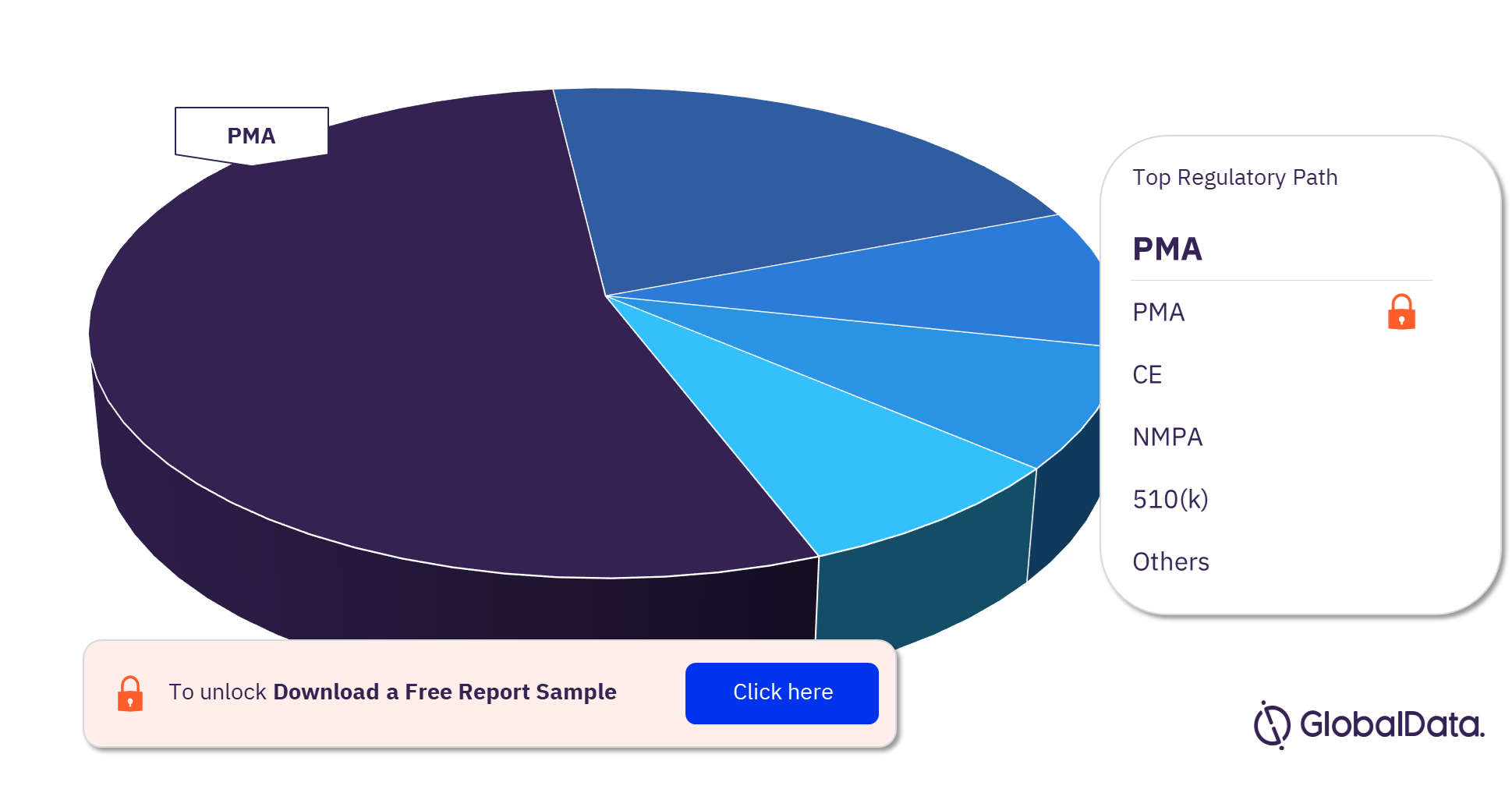
Some of the key regulatory paths in the Prosthetic Heart Valves pipeline market are PMA, CE, NMPA, 510(k), HDE Approvals, ICAC, MDL, Shonin, NIFDS, and HAS. As of April 2023, PMA is the most followed pathway for pipeline products.
Prosthetic Heart Valves Pipeline Market Analysis, by Regulatory Paths, 2023 (%)
For more regulatory paths into the Prosthetic Heart Valves pipeline market, download a free report sample
Prosthetic Heart Valves Pipeline Market - Competitive Landscape
Some of the key companies in the Prosthetic Heart Valves pipeline market are AGEless Biomedical Inc, Anchorvalve, Anteris Technologies Ltd, Artivion Inc, Autus Valve Technologies Inc, Biostage Inc, Boston Children’s Hospital, Colibri Heart Valve LLC, Collagen Solutions Ltd, and Collplant Biotechnologies Ltd.
Anteris Technologies Ltd- Headquartered in Brisbane, Queensland, Australia, Anteris Technologies Ltd, (Anteris Technologies), formerly Admedus Ltd develops, manufactures and distributes medical devices and technologies with focus on tissue engineering and immunotherapies. The company’s product portfolio includes ADAPT technology, a next generation bio scaffold that reengineers xenograft tissue into a pure collagen scaffold and DurAVR heart valve, a 3D single piece aortic valve that creates a wider valve opening and better blood flow.
Artivion Inc- Headquartered in Atlanta, Georgia, the US, Artivion Inc (Artivion), formerly CryoLife Inc, is a medical device company that manufactures and distributes biosurgical devices and implantable human tissues for cardiac and vascular surgical procedures for the treatment of aortic disease. The company’s portfolio enlists preserved human cardiac allografts, vascular allografts, surgical adhesives and sealants, heart valves, bovine pericardium, and mitral chordal repair products, photofix products and Jotec products. The company products serve physicians, cardiac surgeons, hospitals, and medical institutions.
Biostage Inc- Headquartered in Holliston, Massachusetts, the US, Biostage Inc (Biostage) operates as a biotechnology company. The company carries out the business of development and commercialization of regenerated organs for human transplant. It develops bioengineered organ implants based on its Cellframe technology which combines biocompatible scaffold with a patient’s own stem cells to create Cellspan organ implants. Biostage’s pipeline offerings include Cellspan bronchial implants, Cellspan esophageal implants and Cellspan tracheal implants. The company has operations across Germany, the US, Sweden and the UK.
Prosthetic Heart Valves Pipeline Market Report Overview
| Key Territories | The US, Europe, China, India, Israel, Japan, Canada, Singapore, South Korea, and Taiwan |
| Key Segments | Tissue Heart Valves, Transcatheter valve delivery devices, Mechanical Heart Valves, Tissue Sutureless Aortic Valve Replacements, Tissue Sutured Mitral Valve Replacements, Mechanical Sutured Mitral Valve Replacements, and Tissue Sutured Aortic Valve Replacements |
| Key Regulatory Paths | PMA, CE, NMPA, 510(k), HDE Approvals, ICAC, MDL, Shonin, NIFDS, HAS, and BOPA |
| Leading Companies | AGEless Biomedical Inc, Anchorvalve, Anteris Technologies Ltd, Artivion Inc, Autus Valve Technologies Inc, Biostage Inc, Boston Children’s Hospital, Colibri Heart Valve LLC, Collagen Solutions Ltd, and Collplant Biotechnologies Ltd |
Segments Covered in the Report
Prosthetic Heart Valves Pipeline Market Segments Outlook
- Tissue Heart Valves
- Transcatheter valve delivery devices
- Mechanical Heart Valves
- Tissue Sutureless Aortic Valve Replacements
- Tissue Sutured Mitral Valve Replacements
- Mechanical Sutured Mitral Valve Replacements
- Tissue Sutured Aortic Valve Replacements
Prosthetic Heart Valves Pipeline Market Territories Outlook
- The US
- Europe
- China
- India
- Israel
- Japan
- Canada
- Singapore
- South Korea
- Taiwan
Prosthetic Heart Valves Pipeline Market Regulatory Paths Outlook
- PMA
- CE
- NMPA
- 510(k)
- HDE Approvals
- ICAC
- MDL
- Shonin
- NIFDS
- HAS
- BOPA
Scope
- Extensive coverage of the Prosthetic Heart Valves under development
- Reviews details of major pipeline products which includes, product description, licensing and collaboration details and other developmental activities
- Reviews the major players involved in the development of Prosthetic Heart Valves and list all their pipeline projects
- The coverage of pipeline products based on various stages of development ranging from Early Development to Approved / Issued stage
- Provides key clinical trial data of ongoing trials specific to pipeline products
- Recent developments in the segment / industry
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies
- Identify emerging players with potentially strong product portfolio and create effective counter strategies to gain competitive advantage
- Identify and understand important and diverse types of Prosthetic Heart Valves under development
- Develop market-entry and market expansion strategies
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline
- In-depth analysis of the product’s current stage of development, territory and estimated launch date
Anchorvalve
Anteris Technologies Ltd
Artivion Inc
Autus Valve Technologies Inc
Biostage Inc
Boston Children's Hospital
Colibri Heart Valve LLC
Collagen Solutions Ltd
Collplant Biotechnologies Ltd
Colorado State University
Columbia University
CorMatrix Cardiovascular Inc
CroiValve Ltd
Cytograft Tissue Engineering Inc (Inactive)
Daidalos Solutions BV
Department of Biomedical Engineering Columbia University
Edwards Lifesciences Corp
Enlight Medical Technologies (Shanghai) Co Ltd
enVVeno Medical Corp
Florida International University
FluidForm Inc
Foldax Inc
Georgia Institute of Technology
Griffith University
Hocor Cardiovascular Technologies, LLC
Innovator Lab Consultants India Pvt Ltd
Innovia LLC
InspireMD Inc
JC Medical, Inc.
Jinshi Biotechnology (Changshu) Co Ltd
Leman Cardiovascular SA
Lifelet Medical Ltd
Medi-Line S.A.
Medtronic Plc
Meril Life Sciences Pvt Ltd
MicroPort Scientific Corp
National University of Singapore
NaviGate Cardiac Structures, Inc.
NewMed Medical Co Ltd
On-X Life Technologies Inc
Peca Labs Inc
Rambam Health Care Campus
Revivicor Inc
Shanghai Aoliu Medical Technology Co Ltd
Shanghai Hanyu Medical Technology Co Ltd
Sree Chitra Tirunal Institute for Medical Sciences & Technology
St. Jude Medical LLC
Stellenbosch University
Symetis SA
The Charles Stark Draper Laboratory Inc
Thoratec LLC
Thubrikar Aortic Valve, Inc.
TRiCares SAS
TTK Healthcare Ltd
University College London
University of Arizona
University of California
University of California Irvine
University of Cambridge
University of Colorado
University of Florida
University of Nebraska
University of Pennsylvania
University of Pittsburgh Medical Center
University of South Florida
University of Zurich
ValveXchange, Inc. (Inactive)
Vdyne LLC
Venus Haoyue Medtech Ltd
Venus Medtech Hangzhou Inc
VueKlar Cardiovascular Ltd (Inactive)
W. L. Gore & Associates Inc
Xeltis AG
Table of Contents
Table
Figures
Frequently asked questions
-
Which territory has the highest number of pipeline products in the Prosthetic Heart Valves pipeline market as of April 2023?
As of April 2023, the US has the highest number of pipeline products in the Prosthetic Heart Valves pipeline market.
-
Which segment accounted for the largest Prosthetic Heart Valves pipeline market share?
The Tissue Heart Valves accounted for the largest Prosthetic Heart Valves pipeline market share.
-
Which is the most followed regulatory pathway in the Prosthetic Heart Valves pipeline market As of April 2023?
As of April 2023, PMA is the most followed regulatory pathway in the Prosthetic Heart Valves pipeline market.
-
Who are the major players operating in the Prosthetic Heart Valves pipeline market?
Some of the major players operating in the Prosthetic Heart Valves pipeline market are AGEless Biomedical Inc, Anchorvalve, Anteris Technologies Ltd, Artivion Inc, Autus Valve Technologies Inc, Biostage Inc, Boston Children’s Hospital, Colibri Heart Valve LLC, Collagen Solutions Ltd, and Collplant Biotechnologies Ltd.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Prosthetic Heart Valves reports