Quality Control in Medical Devices – Thematic Intelligence
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
The regulatory landscape for medical devices market have undergone major changes in the last couple of years and the ability to navigate these changes is the key to success. Quality control for medical devices includes both a pre-market stage (to obtain regulatory approval) and a post-market stage (to ensure safety and efficacy in a larger test population). Proper navigation of all stages of quality control will continue to be critical to the success of a medical device.
The quality control in medical devices thematic research report highlights key trends impacting the quality control theme. Furthermore, it provides a comprehensive industry analysis and details the major milestones and changes related to regulations in medical devices within the US and Europe.
The Impact of COVID-19 on Medical Device Regulations
An Emergency Use Authorization (EUA) is a way to ensure the availability and use of medical devices and supplies, including vaccines, during public health emergencies. Under a EUA, the FDA can allow the use of products that have not yet been approved in an emergency to diagnose, treat, or prevent serious diseases or conditions. This can be done when certain criteria are met, and no other approved or adequate alternatives are available. On February 4, 2020, the Secretary of the Department of Health and Human Services (HHS) determined that there was a public health emergency that had a significant potential to affect national health. Since then, the FDA has issued EUAs for several medical devices, drugs, and vaccines.
Furthermore, on April 17, 2020, the European Parliament voted to delay the application of the MDR to May 2021. The delay considered the challenges of the COVID-19 pandemic and the need for increased availability of vital medical devices across Europe. As the pandemic increased demands for certain devices, postponing the MDR ensured that the market disruptions for critical devices were limited.
Quality Control in Medical Devices: Key Trends
The key trends that are impacting medical device regulations are artificial intelligence (AI), COVID-19, cybersecurity, digitalization, improved post-market surveillance, and political pressure.
Digitalization: As the digitalization of healthcare continues and becomes even more common, new types of medical devices will continue to be introduced. Some examples of recent digital health tools are wearable tech and mobile health apps. Additionally, more health and wellness-based devices and apps that do not need a prescription are appearing. Health-based mobile apps such as menstrual cycle trackers and calorie trackers are also available.
Quality Control in Medical Devices: Key Regulatory Trends
Some of the key regional trends affecting medical device regulations are listed below:
The Australian Government has undertaken a program of reforms to strengthen the regulation of medical devices in Australia. An action plan for medical devices (Action Plan) was released in 2019. It is a three-part strategy aimed at strengthening Australia’s regulatory system while continuing to be patient-focused, have greater transparency, and increase public confidence in Australia’s medical device regulatory system.
The 510(k), also known as the premarket notification and the premarket approval (PMA), are the major submissions for manufacturers planning to market their products in the US. The key point of the 510(k) is to determine whether the new device is substantially equivalent to a legally marketed device (predicate devices).
Canada has enacted the Forward Regulatory Plan for 2022–2024, which will guide regulation in various areas of government. The plan is a publicly available list with descriptions of planned or anticipated regulatory changes that Health Canada intends to propose or finalize within two years. It may include regulatory initiatives that are planned to come forward over a longer time frame and are thus indicated as long-term.
For more insights on key trends impacting medical device regulations, download a free report sample
Quality Control in Medical Devices – Industry Analysis
The development, production, and use of medical devices are subject to numerous laws, regulations, stringent standards, and certification processes. Several countries that did not have regulatory requirements for medical devices have established such requirements in recent years, while other countries have expanded their existing regulations. Companies are under increasing market pressure to maintain quality while remaining compliant with ever-changing industry regulations.
The quality control in medical devices industry analysis also covers:
- Medical device approvals
- Geographical analysis
- Therapy area analysis
- Social media trends
- Timeline
To know more about the quality control theme in the medical devices industry, download a free report sample
Quality Control in Medical Devices – Key Players
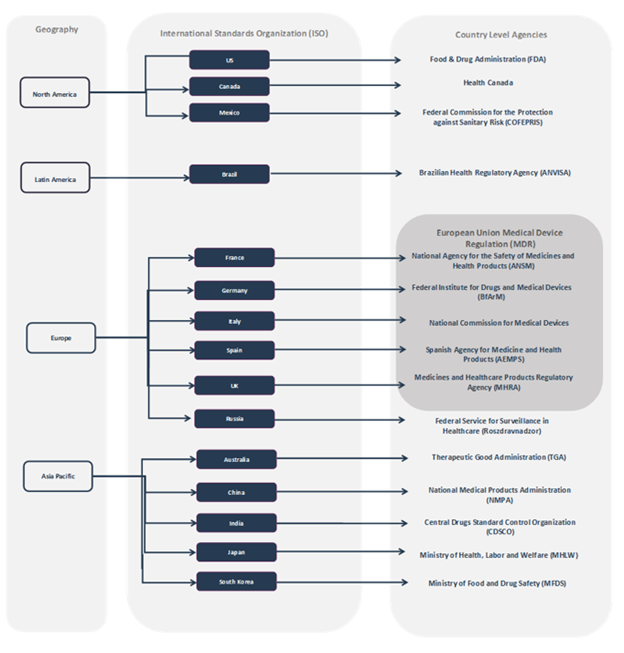
Some of the key medical device regulatory agencies by country are as follows:
- The UK: Medicines and Healthcare Products Regulatory Agency (MHRA)
- Germany: Federal Institute for Drugs and Medical Devices (BfArM)
- France: The National Agency for the Safety of Medicines and Health Products (ANSM)
- Italy: Ministry of Health Directorate General for Medicines and Medical Devices
- Spain: The Spanish Agency of Medicines and Medical Devices (AEMPS)
Medical Device Regulatory Agencies
To know more about the medical device regulatory agencies in each country, download a free report sample
Quality Control in Medical Devices Industry Overview
| Report Pages | 29 |
| Regions Covered | Global |
| Key Trends | Artificial Intelligence, COVID-19, Cybersecurity, Digitalization, Improved Post-Market Surveillance, and Political Pressure |
Scope
- This report is a thematic brief, which identifies those companies most likely to succeed in a world filled with disruptive threats. Inside, we predict how each theme will evolve and identify the leading and disrupting companies.
- The report covers the quality control theme.
Reasons to Buy
- GlobalData’s thematic research ecosystem is a single, integrated global research platform that provides an easy-to-use framework for tracking all themes across all companies in all sectors. It has a proven track record of identifying important themes early, enabling companies to make the right investments ahead of the competition, and securing that all-important competitive advantage.
- Develop and design your corporate strategies through an in-house expert analysis of quality control by understanding the primary ways in which this theme is impacting the medical devices industry.
- Stay up to date on the industry’s major players and where they sit in the value chain.
- Identify emerging industry trends to gain a competitive advantage.
Table of Contents
Frequently asked questions
-
What key trends are impacting medical device regulations?
The key trends that are impacting medical device regulations are artificial intelligence, COVID-19, cybersecurity, digitalization, improved post-market surveillance, and political pressure.
-
Which is the key regulatory agency in the UK?
Medicines and Healthcare Products Regulatory Agency (MHRA) is the key regulatory agency in the UK.
-
Which is the key regulatory agency in Germany?
Federal Institute for Drugs and Medical Devices (BfArM) is the key regulatory agency in Germany.
-
What is the action plan for medical devices undertaken by the Australian government?
The action plan for medical devices is a three-part strategy aimed at strengthening Australia’s regulatory system while continuing to be patient-focused, have greater transparency, and increase public confidence in Australia’s medical device regulatory system
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Medical Devices reports