United States (US) Orthopedic Procedures Count by Segments and Forecast to 2030
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
US Orthopedic Market Report Overview
The total number of Orthopedic Procedures performed in the US was 18,577,953 in 2022. The US Orthopedic Procedures market research report is a comprehensive databook report covering key procedure data on the US Orthopedic Procedures. It also provides key information and data on the procedure volumes for different segments of Orthopedic Procedures conducted in the country during the historical and forecast period.
| Market Size (2022) | 18,577,953 Procedures |
| Forecast Period | 2023-2030 |
| Historical Period | 2015-2022 |
| Key Segments | Orthopedic Bone Cement and Casting Material Procedures, Orthobiologic Procedures, Arthroscopy Procedures, Spinal Surgery Procedures, Knee Reconstruction Procedures, Trauma Fixation Procedures, Hip Replacement Procedures, Cranio Maxillofacial Fixation (CMF) Procedures, Shoulder Replacement Procedures, and Other Joint Reconstruction Procedures |
US Orthopedic Procedures Market Segments
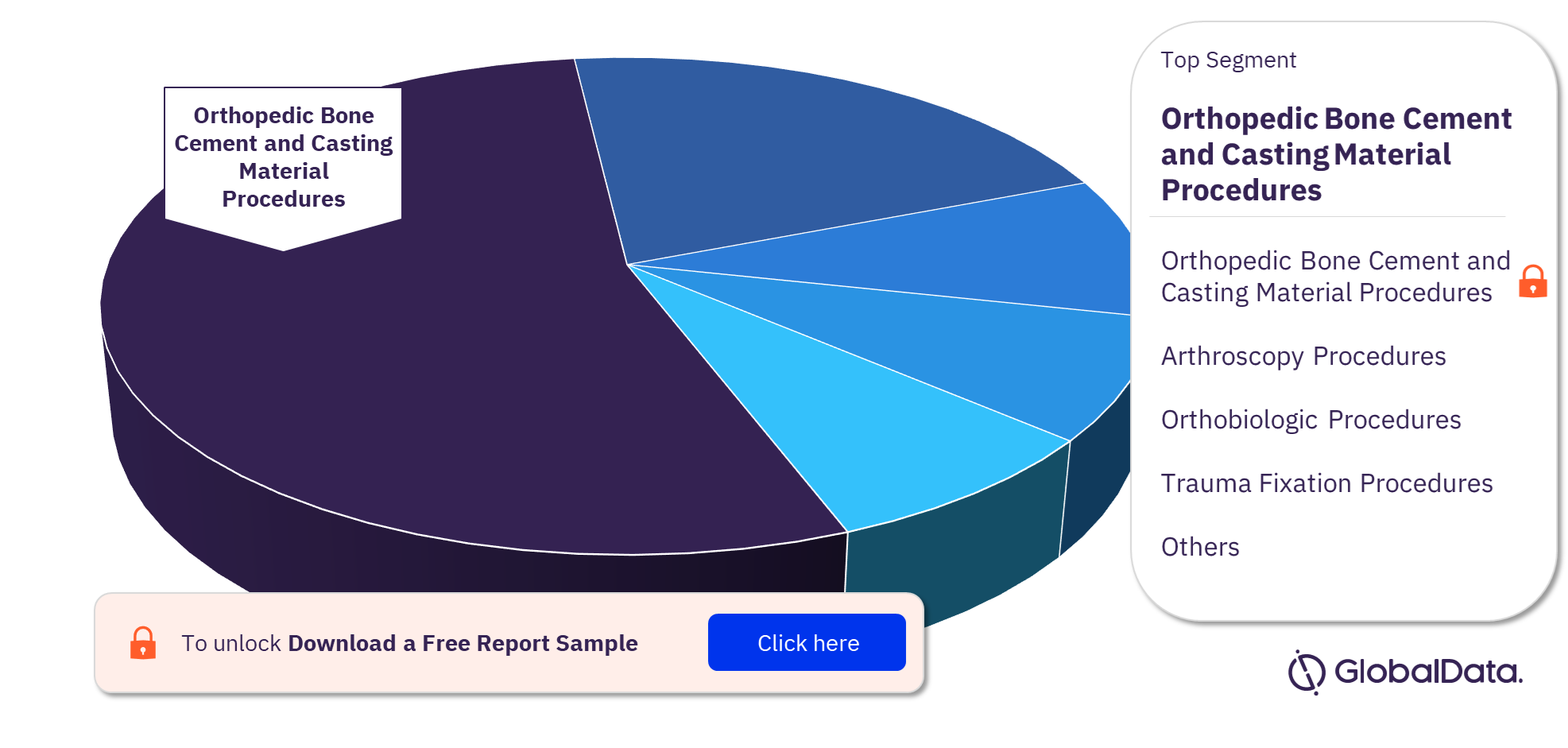
The key segments in the US Orthopedic Procedures market are Orthopedic Bone Cement and Casting Material Procedures, Orthobiologic Procedures, Arthroscopy Procedures, Spinal Surgery Procedures, Knee Reconstruction Procedures, Trauma Fixation Procedures, Hip Replacement Procedures, Cranio Maxillofacial Fixation (CMF) Procedures, Shoulder Replacement Procedures, and Other Joint Reconstruction Procedures. Orthopedic Bone Cement and Casting Material Procedures were the most performed Orthopedic Procedures in the US in 2022.
Orthopedic Bone Cement and Casting Material Procedures: This procedure involves the use of Bone cement and casting materials to fix bone fractures. The procedure is also used as a complement to other devices in procedures such as joint reconstruction and trauma fixation. It can be further segmented into Bone Cement Procedures and Casting Material Procedures. Casting Material Procedures was the leading sub-segment in 2022.
Orthobiologic Procedures: This can be further segmented into Bone Grafts and Substitutes Procedures, Viscosupplementation Procedures, Soft Tissue Biologics Procedures, Bone Growth Stimulators Procedures, and Cartilage Repair Procedures. The number of Bone Grafts and Substitutes Procedures was the highest in 2022 and is expected to remain so during the forecast period.
Arthroscopy Procedures: It is used to diagnose joint problems, surgically repair a joint, and remove loose bones or cartilage. This can be further segmented into Arthroscopic Shaver Procedures, Arthroscopy Implant Procedures, and Arthroscopy Radio Frequency Systems and Wands Procedures. The number of Arthroscopy Procedures performed was the highest in 2022 and is expected to remain so during the forecast period.
US Orthopedic Procedures Market Analysis, by Segments, 2022 (%)
For more insights into the US Orthopedic Procedures market segments, download a free sample report
Segments Covered in the Report
US Orthopedic Procedures Market Segments Outlook (Number of Procedures, 2015-2030)
- Arthroscopy Procedures
- Arthroscopic Shaver Procedures
- Arthroscopy Implant Procedures
- Arthroscopy Radio Frequency Systems and Wands Procedures
- Cranio Maxillofacial Fixation (CMF) Procedures
- Hip Replacement Procedures
- Hip Resurfacing Procedures
- Partial Hip Replacement Procedures
- Primary Hip Replacement Procedures
- Revision Hip Replacement Procedures
- Knee Reconstruction Procedures
- Partial Knee Replacement Procedures
- Primary Knee Replacement Procedures
- Revision Knee Replacement Procedures
- Orthobiologic Procedures
- Bone Grafts and Substitutes Procedures
- Bone Growth Stimulators Procedures
- Cartilage Repair Procedures
- Soft Tissue Biologics Procedures
- Viscosupplementation Procedures
- Orthopedic Bone Cement and Casting Material Procedures
- Bone Cement Procedures
- Casting Material Procedures
- Other Joint Reconstruction Procedures
- Digits Replacement Procedures
- Ankle Replacement Procedures
- Elbow Replacement Procedures
- Wrist Replacement Procedures
- Shoulder Replacement Procedures
- Reverse Shoulder Replacement Procedures
- Total/Primary Shoulder Replacement Procedures
- Revision Shoulder Replacement Procedures
- Partial Shoulder Replacement Procedures
- Shoulder Resurfacing Procedures
- Spinal Surgery Procedures
- Spinal Fusion Procedures
- Spinal Non-Fusion Procedures
- Vertebral Compression Fracture Repair Devices
- Trauma Fixation Procedures
Reasons to Buy
The US Orthopedic Procedures report helps you to develop:
- Business strategies by identifying the key segments poised for strong growth in the future.
- Market-entry and market expansion strategies.
- Develop investment strategies by identifying the key segments expected to register strong growth in the near future.
Table of Contents
Table
Figures
Frequently asked questions
-
What was the US Orthopedic Procedures market size in 2022?
The total number of Orthopedic Procedures conducted in the US was 18,577,953 in 2022.
-
What are the key segments in the US Orthopedic Procedures market?
The key segments in the US Orthopedic Procedures market are Orthopedic Bone Cement and Casting Material Procedures, Orthobiologic Procedures, Arthroscopy Procedures, Spinal Surgery Procedures, Knee Reconstruction Procedures, Trauma Fixation Procedures, Hip Replacement Procedures, Cranio Maxillofacial Fixation (CMF) Procedures, Shoulder Replacement Procedures, and Other Joint Reconstruction Procedures.
-
Which was the leading segment in the US Orthopedic Procedures market in 2022?
Orthopedic Bone Cement and Casting Material Procedures were the most performed Orthopedic Procedures in 2022 in the US.
-
What are the key segments of Orthopedic Bone Cement and Casting Material Procedures in the US Orthopedic Procedures market?
The key segments of Orthopedic Bone Cement and Casting Material Procedures in the US Orthopedic Procedures market are Bone Cement Procedure and Casting Material Procedure.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Orthopedic Devices reports