Outlook for Viral Vector Contract Manufacturing – Gene Therapies, Cell Therapies, and COVID-19 Vaccines
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
The bio/pharmaceutical industry is experiencing a shortage of viral vectors, a component needed to produce gene therapies and gene-modified cell therapies, as well as certain COVID-19 vaccines that require a viral vector, notably those from AstraZeneca and Johnson & Johnson (J&J). This report reveals that viral vector production is limited by insufficient manufacturing capacity, an inefficient manufacturing process, and the requirement for complex specialist facilities.
There are 14 therapies/vaccines that use a viral vector (gene therapies, gene-modified cell therapies, and recombinant vector vaccines) marketed in the EU, Japan, US, and/or UK (post-Brexit).
Fiona Barry, Associate Editor of GlobalData PharmSource, comments: “We predict that this number will soar in the near future. We anticipate that over 100 more gene therapies and gene-modified cell therapies will be approved over approximately the next six years. These therapies will all need viral vectors and will exacerbate the manufacturing shortage.
“A second and more immediate stress on the viral vector supply chain is their use in some COVID-19 vaccines, specifically recombinant vector vaccines. AstraZeneca’s and Johnson & Johnson’s vaccines, as well as some COVID-19 vaccines in use in Russia and China, are of this molecule type.”
Barry continues: “The pharmaceutical industry is working to solve this shortage through scaling up facilities and developing more efficient processes. Top contract manufacturing organisations are investing in more sites, and the industry is working on increasing the efficiency of viral vector production by improving upstream and downstream processes.”
Although these were not the first COVID-19 vaccines to reach the public—mRNA vaccines made by Pfizer/BioNTech and Moderna were first to be administered in North America and Europe under Emergency Use Authorizations (EUAs) or equivalent temporary authorizations—there is a significant need for other vaccine types too. Governments have in aggregate placed orders of approximately 900 million doses for AstraZeneca’s and J&J’s COVID-19 vaccines.
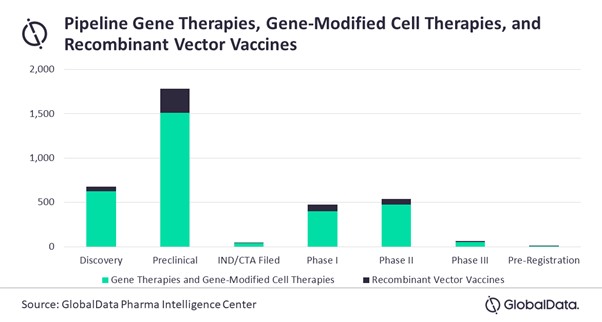
On top of the already approved drugs, there are more than 3,000 gene therapies, gene-modified cell therapies, and recombinant vector vaccines in the development pipeline.
Pipeline Gene Therapies, Gene-Modified Cell Therapies, and Recombinant Vector Vaccines
Barry adds: “Regulatory changes would also ease the viral vector bottleneck. If agencies approve standardized viral platforms that could be used interchangeably by therapy developers, this would speed up development, approval, and technology transfer to CMOs.”
Scope
This report is the companion to GlobalData’s Gene Therapy Market Opportunity for CMOs – 2019 Edition and Cell Therapy Market Opportunity for CMOs – 2018 Edition reports, which described the demand and supply for contract manufacturing in the gene and cell therapy markets. This latest report examines the approvals and manufacturing outlook for three drug molecule types that all require viral vectors in their production: gene therapies, gene-modified cell therapies, and recombinant vector vaccines. The report is critical for benchmarking the CMO industry’s capacity to manufacture these vital vaccines and drugs and forecasting future approvals.
This 62-page report gives important, expert insight you won’t find in any other source. 22 figures and 15 tables throughout the report illustrate major points and trends. This report is required reading for:
• CMO executives who must have deep understanding of the vaccine, cell therapy, and gene therapy marketplace to make strategic planning and investment decisions about viral vector manufacturing.
• Sourcing and procurement executives who must understand crucial components of the supply base in order to make decisions about supplier selection and management.
• Private Equity investors that need a deeper understanding of the market to identify and value potential investment targets.
Reasons to Buy
Detailed view of the geographic distribution of viral vector contract manufacturing facilities worldwide and the proportion belonging to dedicated CMOs and excess capacity manufacturers
Leader and disruptor companies in the viral vector space
Analysis of pipeline and marketed gene therapies, cell therapies, and recombinant vector vaccines, by geography, development stage, and clinical trials
A detailed model forecasting future gene therapy, cell therapy, and recombinant vector vaccine approvals, and associated manufacturing volumes
Contract manufacturing agreements for the active pharmaceutical ingredient (API) component of these vaccines and therapies
Abeona Therapeutics Inc
ActogeniX NV
Adaptimmune Ltd
Adrenas Therapeutics Inc Aspa Therapeutics Inc BridgeBio Pharma Inc
Advanced BioScience Laboratories Inc
Advantagene Inc
Advaxis Inc
Advent Srl
AGC Biologics SpA
Ajinomoto Bio-Pharma Services
Albany Molecular Research Inc
Albert B. Sabin Vaccine Institute Inc
Aldevron LLC
Almac Group Ltd
Altimmune Inc
Amicus Therapeutics Inc
Anchiano Therapeutics Ltd
Aruvant Sciences Inc
ASC Therapeutics Inc
Aslan Pharmaceuticals Ltd
Aspen Pharmacare Holdings Ltd
AstraZeneca KK
AstraZeneca Plc
AstraZeneca Plc University of Oxford
Attwill Medical Solutions
Batavia Biosciences BV
Baxter Biopharma Solutions
Benitec Biopharma Inc
BioCell Corp Ltd
Biological E Ltd
BioReliance Corp
bluebird bio Inc
Boehringer Ingelheim Biopharmaceuticals GmbH Boehringer Ingelheim RCV GmbH & Co KG
BriaCell Therapeutics Corp
Caladrius Biosciences Inc
Candel Therapeutics
Catalent Inc
Cell and Gene Therapy Catapult
Cell Therapies Pty Ltd
Cellectis SA
CELLforCURE
Celsion Corp
Centaur Biopharmaceutical Services Inc
Centre C3i
Chinook Therapeutics Inc
City of Hope
Coalition for Epidemic Preparedness Innovations Public Health Vaccines LLC
Cobra Biologics Ltd
CombiGene AB
Corautus Genetics Inc. (Inactive)
CRISPR Therapeutics AG
Daiichi Sankyo Biotech Co Ltd
Decibel Therapeutics Inc
DiNAQOR AG
Dompe Farmaceutici SpA
Editas Medicine Inc
Eiger BioPharmaceuticals Inc
ElevateBio LLC
Emergent BioSolutions Inc
enGene Inc
Flexion Therapeutics Inc
Forte Biosciences Inc
Fraunhofer-Gesellschaft zur Forderung der Angewandten Forschung eV
Freeline Therapeutics Holdings Plc
Fujifilm Diosynth Biotechnologies USA Inc
Gamaleya Federal Research Center of Epidemiology and Microbiology
Genethon SA Sarepta Therapeutics Inc
Genprex Inc
Genscript Biotech Corp
GenVec LLC
GeoVax Labs Inc
German Center for Infection Research
GlaxoSmithKline Plc
Grand River Aseptic Manufacturing Inc
Halix BV
Handl Therapeutics BV
Helocyte Biosciences Inc
Helocyte, Inc.
Hookipa Pharma Inc
IDT Biologika GmbH
IN8bio Inc
Indapta Therapeutics Inc
Innobation Co Ltd
Insud Pharma
International AIDS Vaccine Initiative
Ion Channel Innovations LLC
iosBio Pharma Ltd
Iovance Biotherapeutics Inc
IVERIC bio Inc
Janssen Pharmaceutica NV
Janssen Pharmaceutica NV Janssen Pharmaceuticals Inc
Janssen Pharmaceuticals Inc
JCR Pharmaceuticals Co Ltd
Jenner Institute
Johnson & Johnson
Juventas Therapeutics Inc
KBI Biopharma Inc
Kite Pharma Inc
Laboratorio Reig Jofre SA
Lentigen Technology Inc
Les Laboratoires Servier SAS
Lonza Group Ltd
Lysogene SAS
Massachusetts General Hospital
MaSTherCell SA
MaxCyte Inc
Merck KGaA
MilliporeSigma
Minaris Regenerative Medicine GmbH
Minaris Regenerative Medicine LLC
Minaris Regenerative Medicine LLC Showa Denko Materials Co Ltd
MTG Biotherapeutics Inc
Mustang Bio Inc
Noga Therapeutics Ltd
Novartis AG
Novartis Gene Therapies
Novartis International AG
Novasep Holding SAS
Novavax Inc
Ology Bioservices Inc
OncoSec Medical Inc
Oncternal Therapeutics
Orchard Therapeutics Plc
Otsuka Holdings Co Ltd
Oxford BioMedica Plc
Oxford-Emergent Tuberculosis Consortium Limited (Inactive)
Passage Bio Inc
Patheon NV
Patheon NV Patheon Viral Vector Services
Pfizer Inc
Pharmasyntez
Poseida Therapeutics Inc
Prevail Therapeutics Inc
ProBioGen AG
Profectus BioSciences Inc
Provecs Medical GmbH
PTC Therapeutics Inc
Rega Institute for Medical Research
RegenxBio Inc
ReiThera Srl
Renova Therapeutics Inc
Richter-Helm BioLogics GmbH & Co KG
Rocket Pharmaceuticals Inc
SanBio Company Limited
Sangamo Therapeutics France SAS
Sanofi
Sarepta Therapeutics Inc
Scenic Biotech BV
Selecta Biosciences Inc
Selexis SA
Sensorion SA
SGS Life Science Services
Sio Gene Therapies Inc
SK Bioscience
Spark Therapeutics Inc
Symbiosis Pharmaceutical Services Ltd
Synlogic Inc
Takara Bio Inc
Taysha Gene Therapies
TCR2 Therapeutics Inc
Themis Bioscience GmbH
T-Knife GmbH
Tonix Pharmaceuticals Holding Corp
Triumvira Immunologics Inc
TTY Biopharm Co Ltd
Ultragenyx Pharmaceutical Inc
UniQure NV
University of Oxford
Valneva SE
Vaxart Inc
VGXI Inc
Vibalogics GmbH
Vigene Biosciences Inc
ViGeneron GmbH
Vir Biotechnology Inc
Viralgen
Vivebiotech SL
Vivet Therapeutics SAS
Wacker Chemie AG
Waisman Biomanufacturing
WuXi AppTec (Shanghai) Co Ltd
WuXi AppTec Co Ltd
Xyphos Biosciences Inc
Yposkesi SAS
Zhejiang Hisun Pharmaceutical Co Ltd
ZIOPHARM Oncology Inc
Table of Contents
Table
Figures
Frequently asked questions
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Pharmaceuticals reports








