Coronavirus Disease 2019 (COVID-19) Impact on Clinical Trials, 2022 Update
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Covid 19 impact on Clinical Trials Market Analysis Report Overview
Since the first case of COVID-19 was diagnosed in Wuhan, China, in December 2019, COVID-19 cases rose rapidly across the globe. On March 11, 2020, World Health Organization (WHO) officially declared that COVID-19 is a pandemic.
Over 1,200 clinical trials worldwide have either been disrupted by COVID-19 due to suspended enrollment or delayed initiation or have been impacted by slow enrollment. Regulatory guidance has been issued for industry, investigators, and institutional review boards on conducting clinical trials during the COVID-19 pandemic, including suggested methods such as virtual visits, phone interviews, self-administration, and remote monitoring These suggestions could help trials that are being met with subject quarantine, travel limitations, clinical site closures, and interrupted supply chains.
The Coronavirus Disease 2019 (COVID-19) impact on clinical trials market research report gives an important update to the previous year with expert analysis on how COVID-19 is impacting clinical trials and the potential move toward decentralized clinical trials (DCTs).
Objectives
The overall objective of the 2022 survey was to update and expand the understanding of the impact of COVID-19 on clinical trials and the industry’s view on adapting to DCTs. DCTs allow the collection of safety and efficacy data from study participants by using a range of digital technologies that require a minimum number of site visits. This can include telemedicine, wearable devices, eConsent, electronic clinical outcome assessments, and electronic health records.
The specific objectives of the 2022 survey were to:
- Update to current perceptions as compared to this same report published in August 2021 and August 2020
- Benchmark the companies’ primary concerns
- Determine the impact of COVID-19 to date on clinical trials
- Assess companies’ future plans to use DCTs
- Track changes to clinical trial strategy and attitudes
Study Design
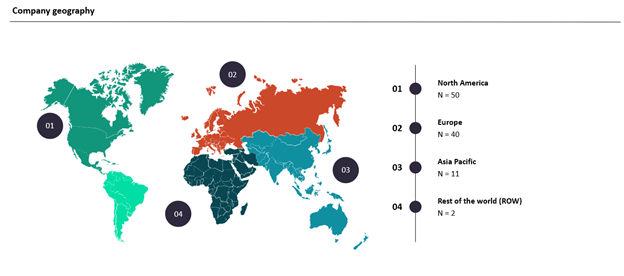
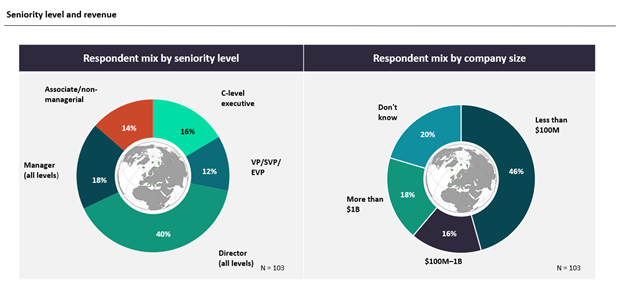
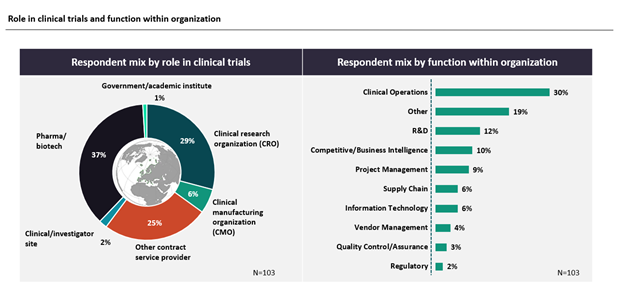
A total of 103 GlobalData Pharma clients and prospects from around the world participated in the seven-minute survey, which was fielded from June 29, 2022, to July 28, 2022. This report includes and is based on industry opinions on COVID-19’s impact on clinical trials and the use of DCTs.
Survey Respondent Mix
For more insights on how COVID-19 is impacting the clinical trial sector, download a free report sample
Key Findings
Clinical trial disruption remains steady and slow enrollment continues to increase: The impact on clinical trial participants and the safety of patients were viewed as major and legitimate concerns. Currently, organizations are utilizing remote patient monitoring to mitigate clinical trial disruptions as a first step, but a plan to move toward DCTs will be the next step.
To know more about key findings, download a free report sample
Reasons to Buy
- Understand companies’ primary concerns about the COVID-19 pandemic.
- Determine the impact of COVID-19 to date on clinical trials.
- Assess companies’ future plans to use decentralized clinical trials.
- Track changes to clinical trial strategy and attitudes.
Table of Contents
Frequently asked questions
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Pharmaceuticals reports