Respiratory Devices Pipeline Report including Stages of Development, Segments, Region and Countries, Regulatory Path and Key Companies, 2022 Update
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Respiratory devices facilitate breathing during respiratory failure and other respiratory disease conditions. This category is further segmented into positive airway pressure devices, non-invasive ventilation masks & circuits, humidifiers, oxygen concentrators, oxygen conservers, ventilators, nebulizers, and respiratory device accessories.
The respiratory devices pipeline market research report provides comprehensive information about the respiratory devices pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
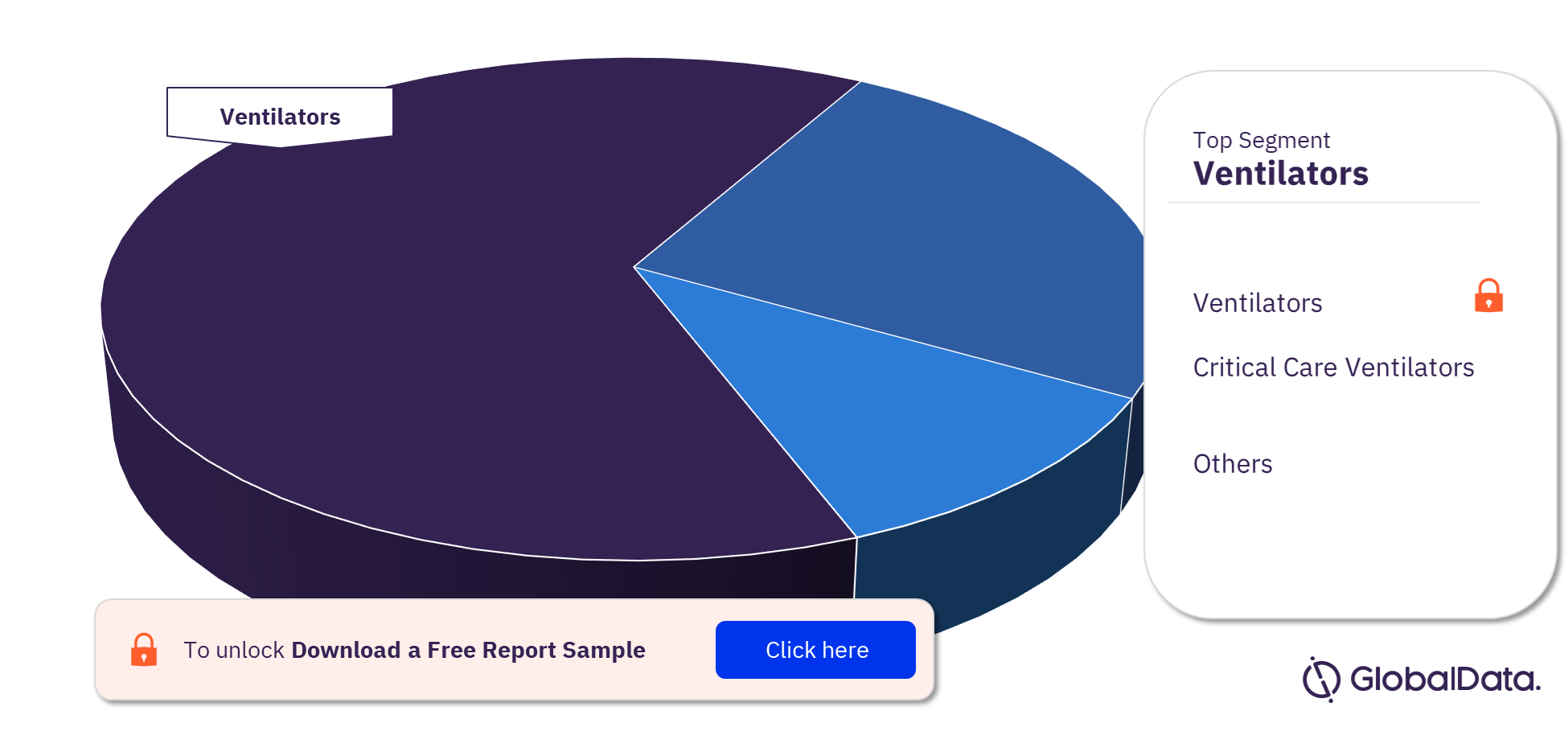
Respiratory Devices Pipeline Products Market Segments
Ventilators, critical care ventilators, artificial lung, nebulizers, non-invasive ventilation masks, portable oxygen concentrators, respiratory assist devices, and respiratory device accessories are some of the key segments in the pipeline product.
Respiratory Devices Pipeline Products Market Analysis, by Segments, 2022(%)
For more segment insights into the respiratory devices pipeline products market, download a free report sample
Respiratory Devices Pipeline Products Market Segmentation, by Territories
Some of the key territories with products in the pipeline are the US, Europe, China, Canada, Australia, Brazil, Japan, India, Chile, Israel, South Korea, and others. As of September 2022, the US has the highest number of products in the pipeline out of them all.
Respiratory Devices Pipeline Products Market Analysis, by Territories, 2022(%)
For more territory insights into the respiratory devices pipeline products market, download a free report sample
Respiratory Devices Pipeline Products Market Segmentation by Key Regulatory Paths
The key regulatory paths followed by the respiratory devices pipeline products market are 510(K), CE, NMPA, HSA, BOPA, ICAC, MDL, Ninsho, and TGA. Most of the products follow the 510(K) pathway to enter the market.
Respiratory Devices Pipeline Products Market Analysis, by Regulatory Paths, 2022 (%)
For more respiratory devices pipeline regulatory path insights, download a free report sample
Competitive Landscape
Some of the leading companies in the respiratory devices pipeline products market are Ablynx NV, Actuated Medical Inc, and ABM Respiratory Care.
Ablynx NV (Ablynx): It is a subsidiary of Sanofi. It is a biopharmaceutical company that focuses on developing Nanobodies, proprietary therapeutic proteins based on single-domain antibody fragments. The company has a portfolio of Nanobody-based therapeutic programs in various vital disease areas, including inflammation, hematology, immuno-oncology and oncology. The company’s proprietary Nanobody platform allows the rapid generation and large-scale production of novel biological therapeutics that have potential to be used in a wide range of human diseases.
Actuated Medical Inc (Actuated Medical): It is a medical device company that develops minimally invasive instruments. The company provides products such as controlled tissue penetration systems, occlusion clearing systems and MRI compatible systems. Its controlled tissue penetration systems use low force, electronically controlled motion to enable smooth insertion of sharps for serial blood sampling procedures. Actuated Medical’s also provides tube clearing solutions that remove clogs in patient. The company’s occlusion clearing systems use mechanical motion to restore patency of tubes in the patient for clearing clogged nasoenteral, gastrostomy, nasogastric, and jejunostomy feeding and decompression tubes. It operates through manufacturing facility in Bellefonte. Actuated Medical is headquartered in Bellefonte, Pennsylvania, the US.
Respiratory Devices Pipeline Products Market Report Overview
| Key Segments | Ventilators, Critical Care Ventilators, Artificial Lung, Nebulizers, Non-Invasive Ventilation Masks, Portable Oxygen Concentrators, Respiratory Assist Devices, and Respiratory Device Accessories |
| Key Territories | The US, Europe, China, Canada, Australia, Brazil, Japan, India, Chile, Israel, South Korea, and Others |
| Key Regulatory Paths | 510(K), CE, NMPA, HSA, BOPA, ICAC, MDL, Ninsho, and TGA |
| Leading Companies | Ablynx NV, Actuated Medical Inc, and ABM Respiratory Care |
Segments Covered in the Report
Respiratory Devices Pipeline Products Market Segments Outlook
- Ventilators, Critical Care Ventilators
- Artificial Lung
- Nebulizers
- Non-Invasive Ventilation Masks
- Portable Oxygen Concentrators
- Respiratory Assist Devices
- Respiratory Device Accessories
Respiratory Devices Pipeline Products Market Territories Outlook
- The US
- Europe
- China
- Canada
- Australia
- Brazil
- Japan
- India
- Chile
- Israel
- South Korea
Scope
This report provides:
- Extensive coverage of respiratory devices under development.
- The report reviews details of major pipeline products which includes, product description, licensing and collaboration details, and other developmental activities
- The report reviews the major players involved in the development of Respiratory Devices and lists all their pipeline projects
- The coverage of pipeline products is based on various stages of development ranging from early development to the approved/issued stage.
- The report provides key clinical trial data of ongoing trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with a potentially strong product portfolio and create effective counterstrategies to gain a competitive advantage.
- Identify and understand important and diverse types of Respiratory Devices under development.
- Develop market-entry and market-expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date.
ABM Respiratory Care
Actuated Medical Inc
Adherium Ltd
Advanced Medical Electronics Corp
Advanced Respiratory Technologies LLC
Aeon Research & Technology
Aero Therapeutics Inc
Aerobiosys innovations Pvt Ltd
Aeronics USA LLC
AFT Pharmaceuticals Ltd
AgVa Healthcare Pvt Ltd
Airing LLC
Ajeenkya DY Patil University
All India Institute of Medical Sciences
Aloro Medical AB
ALung Technologies Inc
Anemed LLC
ArcelorMittal India Pvt Ltd
Armadilla Ltd
Ashok Leyland Ltd
Auckland University of Technology
Aura Medical
Babcock International Group Plc
Baby's Breath Ltd
Baxter Academy for Technology and Science
Baxter Healthcare Corp
Beijing Dehaier Medical Technology Co Ltd
Belluscura Plc
Ben-Gurion University of the Negev
Bessel LLC
Beyond Air Inc
Bhabha Atomic Research Centre
Bhagwati Products Ltd
Blucore Pty Ltd
Braile Biomedica Ltda
Breethe, Inc.
Bridgesource Medical Corp
Broncus Medical Inc
Cambridge Consultants Ltd
Carlos III University of Madrid
Case Western Reserve University
Certus Critical Care Inc
Chandigarh University
Chirana Medical, A.S.
Cionic Inc
Cleveland Medical Devices Inc
Columbia Life Systems Inc
Columbia University
Combilift Material Handling Solutions
Compact Medical Solutions LLC
ConzumeX Industries Pvt Ltd
Corestone Biosciences (Beijing) Co Ltd
Coridea, LLC
Creare LLC
CSA Medical Inc
Cubic Corp
Darmstadt University of Applied Sciences
Deca-Medics Inc
Delta chase LLC
DesignWise Medical Inc (Inactive)
Discover Medical Device Ltd (Inactive)
Don Bosco Technical College
Dragerwerk AG & Co KGaA
Duke University
Dyson Ltd
enmodes GmbH
Eolo Medical Inc
European Organization for Nuclear Research
Evolving Medical Solutions Inc
Filtara, Inc.
First Wave Technologies Inc
Fisher & Paykel Healthcare Corporation Ltd
FlexSys Inc
Formlabs Inc
Fracktal Works Pvt Ltd
G H Raisoni College of Engineering
Gas N2itrogen SL
GE Healthcare
Georgetown University
Georgia Institute of Technology
Getinge AB
Gilero LLC
Griffith University
GRS India Corp
Hadassah Medical Center
Hamilton Medical AG
HCmed Innovations Co Ltd
Hill-Rom Holdings Inc
Hyundai Motor Co
IBD Italian Biomedical Devices Srl
Imperial College London
Inali Foundation
Indeema Fibres Pvt Ltd
Indian Institute of Science
Indian Institute of Science Education & Research
Indian Institute of Technology Bhubaneswar
Indian Institute of Technology Bombay
Indian Institute of Technology Delhi
Indian Institute of Technology Dhanbad
Indian Institute of Technology Guwahati
Indian Institute of Technology Hyderabad
Indian Institute of Technology Jammu
Indian Institute of Technology Kharagpur
Indian Institute of Technology Palakkad
Indian Institute of Technology Roorkee
Indian Institute of Technology Ropar
Indian Institute of Technology Tirupati
Indian Institute of Technology, Kanpur
Inofab Health Technologies
Inogen Inc
Inspiration Healthcare Group plc
Institute for Transformative Technologies
Integrated Polytechnic Regional College Kigali
IntelliBreathe Ltd.
Ironstone Professional Development
Jamboxx Inc
Jesse Brown VA Medical Center
Johns Hopkins University
Kapurthala Railway Coach Factory
KeepMED Ltd
Kepley BioSystems Inc
Khalifa University
Kiira Motors Corp
K-One Technology Berhad
Koninklijke Philips NV
Koronis Biomedical Technologies Corporation
Kreator 3d Printer Solutions Pvt Ltd
Kritikare India Pvt Ltd
Kwame Nkrumah University of Science and Technology
Kwivik Therapeutics Inc
Lawrence Livermore National Laboratory
Lawson Health Research Institute
Lehigh University
Liberate Medical LLC
LifeCan Medical Ltd
LifeServe Innovations, LLC
Lifewave Biomedical Inc
Ligand Innovation Global Inc
Lund University
Lundquist
Lung Biotechnology PBC
Lungpacer Medical Inc
Mahindra & Mahindra Ltd
MAQUET Cardiopulmonary AG
Marquette University
Massachusetts Institute of Technology
Materialise NV
MC3 Inc
McGowan Institute for Regenerative Medicine
Medex
Medicom Innovation Partner a/s
Medivations Pty Ltd
Medovate Ltd
Medsimlab Lda
Mergenet Medical, Inc.
Metamason Inc
MFM LLC
MG Motor India Pvt Ltd.
Michigan State University
Ministry of Energy and Mineral Resources, Indonesia
Minnesota Health Solutions Corporation
Miromatrix Medical Inc
Monivent AB
MonuMedical LLC
mPhase Technologies Inc
Mullen Technologies Inc
Nanyang Technological University
National Aeronautics and Space Administration
National Institute of Technology Durgapur
National Institute of Technology Karnataka
Naval Sea Logistics Center
Neonatal Rescue LLC
Netsensing Technology Sarl
Nihon Kohden Corp
Nob Hill Therapeutics Inc
Nocca Robotics Pvt Ltd
Northwestern University
Nota Laboratories LLC
Nova Scotia Health Authority
NovaResp Technologies Inc
Noveko International Inc
Novlead Biotechnology Co Ltd
NumaVent
Nu-Med Plus Inc
Nuvaira Inc
NVIDIA Corp
Omron Healthcare Co Ltd
Omron Healthcare Inc
OneBreath Inc.
Oregon Health & Science University
OscillaVent Inc
Padmaseetha Technologies Pvt Ltd
PARI GmbH
Parion Sciences Inc
Pavad Medical
Pennsylvania State University
Philips Healthcare
Philips Respironics Inc
Polytechnic University Of Madrid
Polytechnic University of Valencia
Pontifical Catholic University of Peru
Portaero Inc
Postgraduate Institute of Medical Education and Research
PremieBreathe
Proactive Life Inc
Prospiria Inc
Pulmonx Corp
Quantaira Health
Radicare (M) Sdn Bhd
Rafina Innovations Inc
Rathinam Group of Institutions
Reactive Innovations, LLC
RemSleep Holdings Inc
ResMed Inc
RespiNova Ltd
Respira Ltd
Respira Technologies Inc
ResusRight Pty Ltd
Rethink Respironics Inc
Rethink Technologies LLC
Rhinomed Ltd
Rice University
RightAir LLC
RK University
RMIT University
Sagentia Ltd
SaiOx Inc
Scottish Health Innovations Ltd
Sensory Innovation Solutions
Shanghai Asclepius Meditec Inc
Sleep Secure LLC
SleepUp Ltd
Smart Oxygen Solutions, Inc.
Smith & Nephew Plc
Smith College
SolAeroMed Inc
Southern Railway
Spyras Ltd
Sree Chitra Tirunal Institute for Medical Sciences & Technology
Staffordshire University
Stanford University
STEMrev Refineries Pvt Ltd
Stephan Design and Engineering Ltd
Steros GPA Innovative SL
Stimit AG
Stirling Pharma Inc
Stogger BV
SynDermix AG
Tda Research Inc.
Teal Bio
Tel Aviv University
Teleflex Inc
Tenax Therapeutics Inc
Texas Tech University
The Chaim Sheba Medical Center
The Charles Stark Draper Laboratory Inc
TigerLAB
TKM College of Engineering-Kerala University
TMC HealthCare
Tolomatic Inc
Toyota Motor Corp
TVP Health
TVS Group
U.S. Ann Arbor Healthcare System
Universidad Nacional de Colombia
Universidad Panamericana
Universiti Teknologi MARA
University at Buffalo
University College Dublin
University Hospital Freiburg
University of Aberdeen
University of Antioquia
University of Arizona
University of Barcelona
University of Botswana
University of Calgary
University of California Davis
University of California Los Angeles
University of California San Diego
University of Cincinnati
University of Connecticut
University of Florida
University of Illinois at Chicago
University of Michigan
University of Michigan Pediatric Device Consortium
University of Missouri
University of Pittsburgh
University of Rochester
University of South Florida
University of Tennessee
University of Texas at San Antonio
University of Texas Medical Branch at Galveston
University of Utah
University of Warwick
Uptake Medical Technology Inc
Vapotherm Inc
Vectura Group Plc
Ventec Life Systems Inc
Ventis Medical Inc
Ventora Medical Pty Ltd
Vero Biotech LLC
Verona Pharma Plc
Veterans Health Administration
Vexos Corp
Villanova University
Vincent Medical Holdings Ltd
Vivaspire Inc
Vrije University Brussel
Wake Forest Baptist Medical Center
Walter Reed National Military Medical Center
Wellinks Inc
WellO2 Oy
Western Health Pty Ltd
Whalen Biomedical Inc
Wise Ally International Holdings Ltd
Worcester Polytechnic Institute
X-Biomedical Inc
X-COR Therapeutics
Xenios AG
XN Health Inc
Xo Thermix Medical, Inc (Inactive)
Z-Cube Srl
Zen Technologies Ltd
Zoll Medical Corp
Table of Contents
Table
Figures
Frequently asked questions
-
What are the key territories in the respiratory devices pipeline products market?
The US, Europe, China, Canada, Australia, Brazil, Japan, India, Chile, Israel, South Korea, and others are some of the key territories with products in the pipeline.
-
What are the key segments in the respiratory devices pipeline products market?
Ventilators, critical care ventilators, artificial lung, nebulizers, non-invasive ventilation masks, portable oxygen concentrators, respiratory assist devices, and respiratory device accessories are some of the key segments in the pipeline.
-
What are the key regulatory paths of the respiratory devices pipeline products market?
Some of the key regulatory paths followed by the respiratory devices pipeline products market are 510(K), CE, NMPA, HSA, BOPA, ICAC, MDL, Ninsho, and TGA.
-
Which are the leading companies in the respiratory devices pipeline products market?
Some of the leading companies in the respiratory devices pipeline products market are Ablynx NV, Actuated Medical Inc, and ABM Respiratory Care.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Anesthesia and Respiratory Devices reports