21 Dec, 2020 49% of clinical trial disruption due to slow enrollment
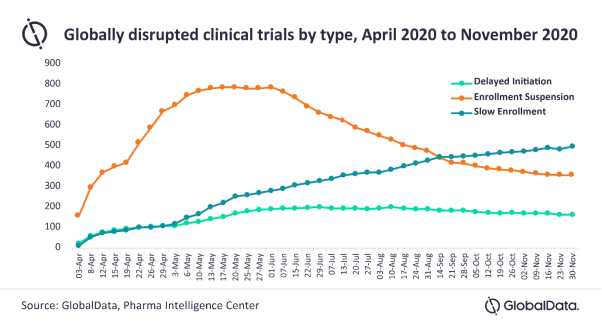
Posted in PharmaAs of November 30, 2020, the top reason for disrupted clinical trials is slow enrollment, which is causing delays in 49% of trials. The other main reasons for disruptions are enrollment suspension at 35%, and delayed initiation at 16%, says GlobalData, a leading data and analytics company.
Scotty Chung-Siu, Pharma Analyst at GlobalData, comments: “Although suspended trials have begun to recruit participants, delays in trial initiation and slow recruitment continue.”
Since June 1, there has been a slow but progressive decline in the number of clinical trial disruptions. The fastest rate of trial disruption was between early April and mid-May, and the number of trials disrupted by COVID-19 peaked in the first week of June.
Chung-Siu concludes: “New vaccines that are being approved for the treatment of COVID-19 provide hope that this will change the dynamic of the global pandemic and decrease the number of disrupted clinical trials in the near future.”
