Aesthetic Devices – Pipeline Products by Stage of Development 33
Aesthetic Devices – Pipeline Products by Segment 34
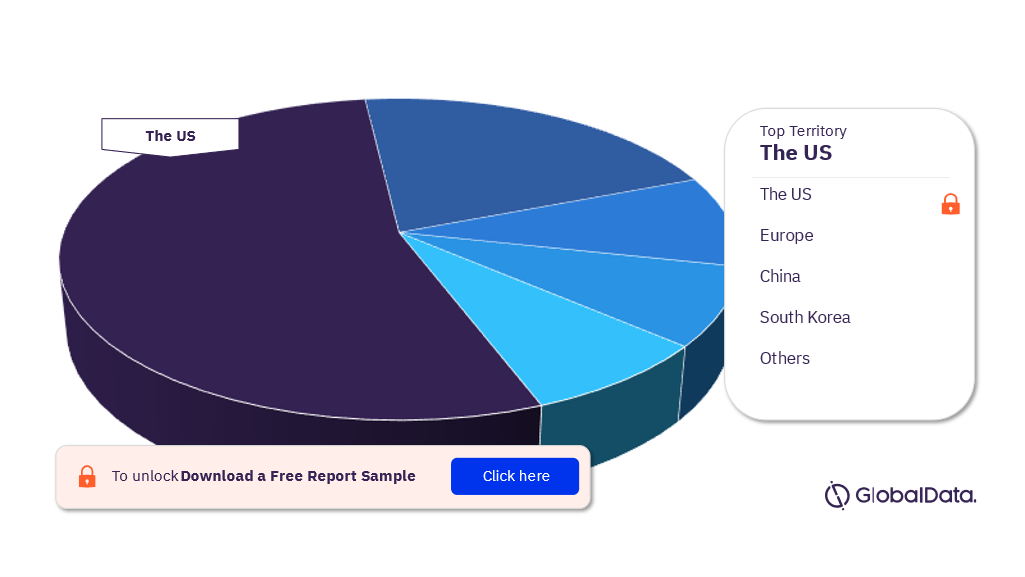
Aesthetic Devices – Pipeline Products by Territory 36
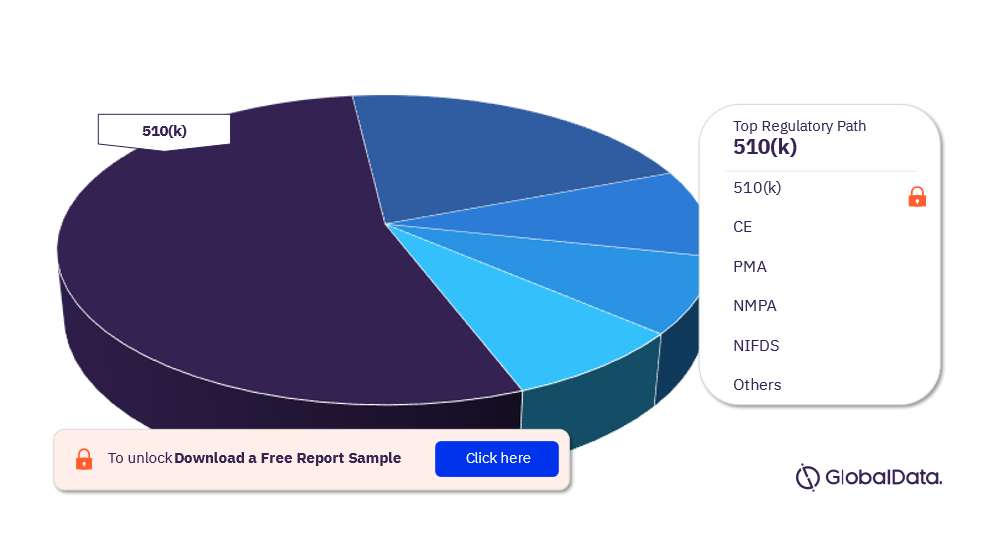
Aesthetic Devices – Pipeline Products by Regulatory Path 38
Aesthetic Devices – Pipeline Products by Estimated Approval Date 39
Aesthetic Devices – Ongoing Clinical Trials 40
Aesthetic Devices Companies – Pipeline Products by Stage of Development 41
Aesthetic Devices – Pipeline Products by Stage of Development 48
3D Bio Corp Pipeline Products & Ongoing Clinical Trials Overview 56
3D Printed Breast Implant – Lupectomy Reconstruction – Product Status 56
3D Printed Breast Implant – Lupectomy Reconstruction – Product Description 56
AbbVie Inc Pipeline Products & Ongoing Clinical Trials Overview 57
Hyaluronic Acid Filler – Skin – Product Status 57
Hyaluronic Acid Filler – Skin – Product Description 57
Acro Biomedical Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 58
ACROFILL – Product Status 58
ACROFILL – Product Description 58
Advanced Aesthetic Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 59
Algeness DF – Product Status 59
Algeness DF – Product Description 59
Algeness HD – Product Status 60
Algeness HD – Product Description 60
Algeness LD – Product Status 60
Algeness LD – Product Description 61
Algeness VL – Product Status 61
Algeness VL – Product Description 61
Advanced Aesthetic Technologies Inc – Ongoing Clinical Trials Overview 62
Algeness VL – A Pivotal Clinical Study to Evaluate the Effectiveness and Safety of Algeness VL for the Correction of Moderate to Severe Nasolabial Folds 63
Algeness VL – A Prospective, Multi-center, Randomized, Controlled, Single-blind Study of the Safety and Effectiveness of Algeness DF 3.5% Deep Volumizing Filler to Correct Age-related Volume Deficit in the Mid-face 63
Aeon Astron Corporation Pipeline Products & Ongoing Clinical Trials Overview 64
BioDermal Filler – Product Status 64
BioDermal Filler – Product Description 64
Prosthetic Chin – Product Status 65
Prosthetic Chin – Product Description 65
Aesthetic Medical Device Pipeline Products & Ongoing Clinical Trials Overview 66
Alluris – Product Status 66
Alluris – Product Description 66
Skinlumina – Product Status 67
Skinlumina – Product Description 67
SkinTechMD – Product Status 67
SkinTechMD – Product Description 68
Aesthetics BioMedical, Inc. Pipeline Products & Ongoing Clinical Trials Overview 69
Vivace Ultra – Product Status 69
Vivace Ultra – Product Description 69
Aesthetics Point Ltd. Pipeline Products & Ongoing Clinical Trials Overview 70
Juvence Implant – Product Status 70
Juvence Implant – Product Description 70
AirXpanders, Inc. Pipeline Products & Ongoing Clinical Trials Overview 71
Smooth Shell AeroForm Tissue Expander System – Product Status 71
Smooth Shell AeroForm Tissue Expander System – Product Description 71
Allergan Aesthetics Pipeline Products & Ongoing Clinical Trials Overview 72
AAM SubQ Body Filler – Product Status 72
AAM SubQ Body Filler – Product Description 73
ADM Subdermal Body Filler – Product Status 73
ADM Subdermal Body Filler – Product Description 73
Elastagen – Acne Scars – Product Status 73
Elastagen – Acne Scars – Product Description 74
Elastagen – Skin Quality – Product Status 74
Elastagen – Skin Quality – Product Description 74
Eplica Silk – Product Status 74
Eplica Silk – Product Description 75
HA Threads – Product Status 75
HA Threads – Product Description 75
Inspira TF2 – Breast Augmentation – Product Status 75
Inspira TF2 – Breast Augmentation – Product Description 76
Juvederm – Decolletage – Product Status 76
Juvederm – Decolletage – Product Description 76
Juvederm – Hands – Product Status 76
Juvederm – Hands – Product Description 77
Juvederm – Neck Lines – Product Status 77
Juvederm – Neck Lines – Product Description 77
Juvederm RejuveCross – Deep Wrinkles – Product Status 77
Juvederm RejuveCross – Deep Wrinkles – Product Description 78
Juvederm RejuveCross – Etched Lines Wrinkles – Product Status 78
Juvederm RejuveCross – Etched Lines Wrinkles – Product Description 78
Juvederm RejuveCross Volumize – Cheek Augmentation – Product Status 78
Juvederm RejuveCross Volumize – Cheek Augmentation – Product Description 79
Juvederm Voluma – Nose – Product Status 79
Juvederm Voluma – Nose – Product Description 79
Juvederm Voluma – Temple – Product Status 79
Juvederm Voluma – Temple – Product Description 80
Juvederm Voluma Global – Malar Augmentation – Product Status 80
Juvederm Voluma Global – Malar Augmentation – Product Description 80
Larger Inspira TE – Product Status 80
Larger Inspira TE – Product Description 81
Phoenix – Breast Augmentation – Product Status 81
Phoenix – Breast Augmentation – Product Description 81
rhCollagen Dermal Filler – Product Status 81
rhCollagen Dermal Filler – Product Description 82
SeriGel – Product Status 82
SeriGel – Product Description 82
Skin Quattro Device – Product Status 83
Skin Quattro Device – Product Description 83
Allergan Aesthetics – Ongoing Clinical Trials Overview 84
Juvederm Voluma Global – Malar Augmentation – A Prospective, Multi-center, Randomized, Controlled, Single-blind Study of the Safety and Effectiveness of Algeness DF 3.5% Deep Volumizing Filler to Correct Age-related Volume Deficit in the Mid-face 85
Juvederm Voluma Global – Malar Augmentation – Clinical, Instrumental and Histological Evaluation of the Combined Use of Onabotulinumtoxin A and Hyaluronic Acid Fillers in Patients with Facial Paralysis 85
Juvederm Voluma – Temple – A Multicenter, Evaluator-blinded, Randomized, No-treatment Controlled Study to Evaluate the Safety and Effectiveness of Juvederm Voluma with Lidocaine for Correction of Temple Hollowing in Chinese Population 86
Almirall Ltd Pipeline Products & Ongoing Clinical Trials Overview 87
Hyaluronic Acid Facial Filler – Product Status 87
Hyaluronic Acid Facial Filler – Product Description 87
Ambicare Health Ltd Pipeline Products & Ongoing Clinical Trials Overview 88
Ambulight Rejuvenate – Product Status 88
Ambulight Rejuvenate – Product Description 88
AngioDynamics Inc Pipeline Products & Ongoing Clinical Trials Overview 89
Next Generation Laser – Product Status 89
Next Generation Laser – Product Description 89
Apex Medical Device Design LLC Pipeline Products & Ongoing Clinical Trials Overview 90
DefyGravity – Product Status 90
DefyGravity – Product Description 90
Arula Technolgies Pipeline Products & Ongoing Clinical Trials Overview 91
Breast Prosthesis – Product Status 91
Breast Prosthesis – Product Description 91
Asymmetric Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 92
Asymmetric Laser Tool – Product Status 92
Asymmetric Laser Tool – Product Description 92
B2M Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 93
Mesenteric Fat Cryolipolysis Device – Product Status 93
Mesenteric Fat Cryolipolysis Device – Product Description 93
Bausch Health Companies Inc Pipeline Products & Ongoing Clinical Trials Overview 94
SOF – 010: Skin Resurfacing – Product Status 94
SOF – 010: Skin Resurfacing – Product Description 94
SOM – 010: Vascular Lesions – Product Status 95
SOM – 010: Vascular Lesions – Product Description 95
SOT – 010: Fine Lines & wrinkles – Product Status 95
SOT – 010: Fine Lines & wrinkles – Product Description 95
SOT – 011: Fine Lines & Wrinkles – Product Status 96
SOT – 011: Fine Lines & Wrinkles – Product Description 96
XUA – 001: Hair Removal – Product Status 96
XUA – 001: Hair Removal – Product Description 96
Becton Dickinson and Co Pipeline Products & Ongoing Clinical Trials Overview 97
Endo Laser – Product Status 97
Endo Laser – Product Description 97
Beijing Meibo Pharmaceutical Biotechnology Development Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 98
ECM Composite Collagen – Product Status 98
ECM Composite Collagen – Product Description 98
Beijing MeiYan KongJian Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 99
Complex Collagen Filler (Type III) – Product Status 99
Complex Collagen Filler (Type III) – Product Description 99
First Generation PLLA Filler (Type III) – Product Status 100
First Generation PLLA Filler (Type III) – Product Description 100
Second Generation PCL Filler (Type III) – Product Status 100
Second Generation PCL Filler (Type III) – Product Description 101
BellaSeno GmbH Pipeline Products & Ongoing Clinical Trials Overview 102
Senella Scaffold – Product Status 102
Senella Scaffold – Product Description 102
BellaSeno GmbH – Ongoing Clinical Trials Overview 103
Senella Scaffold – Clinical Trial Evaluating Feasibility and Safety of Medical-grade Polycaprolactone-PCL Breast Scaffold Implantation with Autologous Fat Grafting for Breast Implant Revision and Congenital Defect Correction Surgery 104
BellaSeno Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 105
3D Printed Pectus Scaffold Implant – Pectus Excavatum – Product Status 105
3D Printed Pectus Scaffold Implant – Pectus Excavatum – Product Description 105
BellaSeno Pty Ltd – Ongoing Clinical Trials Overview 106
3D Printed Pectus Scaffold Implant – Pectus Excavatum – A Clinical Trial Evaluating Medical-Grade Polycaprolactone-PCL Pectus Scaffold Implantation with Autologous Fat Grafting for Pectus Excavatum Camouflage 107
3D Printed Pectus Scaffold Implant – Pectus Excavatum – Clinical Trial Evaluating Medical-grade Polycaprolactone-PCL Pectus Scaffold Implantation With Autologous Fat Grafting for Pectus Excavatum Camouflage (IT) 107
Bimini Health Technologies Pipeline Products & Ongoing Clinical Trials Overview 108
Dermapose Fat Removal and Transfer System – Product Status 108
Dermapose Fat Removal and Transfer System – Product Description 108
Kerastem – Product Status 109
Kerastem – Product Description 109
Puregraft Boost Adipose Micronizer – Product Status 109
Puregraft Boost Adipose Micronizer – Product Description 110
Puregraft Microfat – Product Status 110
Puregraft Microfat – Product Description 110
BioPlus Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 111
Biological Breast – Product Status 111
Biological Breast – Product Description 111
Hyaluronic Acid Filler – Bio-cartilage Replacement – Product Status 112
Hyaluronic Acid Filler – Bio-cartilage Replacement – Product Description 112
Hyaluronic Acid Filler – Bladder Tissue Repair – Product Status 112
Hyaluronic Acid Filler – Bladder Tissue Repair – Product Description 113
Biosculpture Technology, Inc. Pipeline Products & Ongoing Clinical Trials Overview 114
Airbrush Liposculptor IIE – Product Status 114
Airbrush Liposculptor IIE – Product Description 114
Airbrush Liposculptor IIIE – Product Status 115
Airbrush Liposculptor IIIE – Product Description 115
Biostruxs LLC Pipeline Products & Ongoing Clinical Trials Overview 116
BioBreast Scaffold – Product Status 116
BioBreast Scaffold – Product Description 116
Bioxis Pharmaceuticals Pipeline Products & Ongoing Clinical Trials Overview 117
Cytosmile – Product Status 117
Cytosmile – Product Description 117
MTI-12 – Product Status 118
MTI-12 – Product Description 118
MTI-12 – Surgical Repair – Product Status 118
MTI-12 – Surgical Repair – Product Description 119
BMG Pharma SpA Pipeline Products & Ongoing Clinical Trials Overview 120
SGA300 – Product Status 120
SGA300 – Product Description 120
SGA302 – Product Status 121
SGA302 – Product Description 121
SGA320 – Product Status 121
SGA320 – Product Description 121
Bmi Korea Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 122
BMI4002 – Product Status 122
BMI4002 – Product Description 122
Boston Scientific Corp Pipeline Products & Ongoing Clinical Trials Overview 123
Next Gen Greenlight Fiber System – Product Status 123
Next Gen Greenlight Fiber System – Product Description 123
Starfire Laser – Product Status 124
Starfire Laser – Product Description 124
Candela Corp Pipeline Products & Ongoing Clinical Trials Overview 125
Pneumatic Skin Fattening For Telangectasia – Product Status 125
Pneumatic Skin Fattening For Telangectasia – Product Description 125
CellPraxis Pipeline Products & Ongoing Clinical Trials Overview 126
Lipage – Product Status 126
Lipage – Product Description 126
Celltrix AB Pipeline Products & Ongoing Clinical Trials Overview 127
Credurance – Product Status 127
Credurance – Product Description 127
Clearbridge Health Ltd Pipeline Products & Ongoing Clinical Trials Overview 128
Nanofibrous Scaffold – Product Status 128
Nanofibrous Scaffold – Product Description 128
ClearIt LLC Pipeline Products & Ongoing Clinical Trials Overview 129
ERASER System – Product Status 129
ERASER System – Product Description 129
Collplant Biotechnologies Ltd Pipeline Products & Ongoing Clinical Trials Overview 130
3D Bioprinted Regenerative Breast Implant – Product Status 130
3D Bioprinted Regenerative Breast Implant – Product Description 131
3D Bioprinted Regenerative Soft Tissue Matrix – Product Status 131
3D Bioprinted Regenerative Soft Tissue Matrix – Product Description 131
Injectable Breast Implant – Product Status 132
Injectable Breast Implant – Product Description 132
rhCollagen Based Photocurable Regenerative Dermal Filler – Product Status 132
rhCollagen Based Photocurable Regenerative Dermal Filler – Product Description 133
rhCollagen Based Soft Tissue Filler – Product Status 133
rhCollagen Based Soft Tissue Filler – Product Description 133
Cutera Inc Pipeline Products & Ongoing Clinical Trials Overview 134
enlighten Multi-Wavelength – Tattoo Removal – Product Status 134
enlighten Multi-Wavelength – Tattoo Removal – Product Description 134
Cutera Inc – Ongoing Clinical Trials Overview 135
enlighten Multi-Wavelength – Tattoo Removal – A Multi-center Pilot Study of a Novel Multi-wavelength Laser for Tattoo Removal 136
Cytrellis Biosystems Inc Pipeline Products & Ongoing Clinical Trials Overview 137
Micro-Excision Device – Product Status 137
Micro-Excision Device – Product Description 137
Cytrellis Biosystems Inc – Ongoing Clinical Trials Overview 138
Micro-Excision Device – A Prospective, Multi-center, Pilot Study to Evaluate the Safety and Efficacy of the Cytrellis Micro-coring Device for the Treatment of Scars 139
Micro-Excision Device – A Prospective, Multi-center, Pivotal Study to Evaluate the Safety and Efficacy of the Cytrellis Micro-Coring Device for the Treatment of Moderate to Severe Facial Wrinkles 139
Delft University of Technology Pipeline Products & Ongoing Clinical Trials Overview 140
DPS Controlled Laser Coagulation Needle – Product Status 140
DPS Controlled Laser Coagulation Needle – Product Description 140
Dexlevo Inc Pipeline Products & Ongoing Clinical Trials Overview 141
Gouri – Product Status 141
Gouri – Product Description 141
Dongkook Pharmaceutical Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 142
DKB-119 – Product Status 142
DKB-119 – Product Description 142
DKB-133 – Product Status 143
DKB-133 – Product Description 143
DKM-410 – Product Status 143
DKM-410 – Product Description 144
DKM-423 – Product Status 144
DKM-423 – Product Description 144
Emory University Pipeline Products & Ongoing Clinical Trials Overview 145
Nasolabial Fold Implant – Product Status 145
Nasolabial Fold Implant – Product Description 145
Endomimetics LLC Pipeline Products & Ongoing Clinical Trials Overview 146
Silicone Implant – Product Status 146
Silicone Implant – Product Description 146
EP Global Communications Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 147
MEMS Nerve Ablation Device – Facial Glabellar Frowning – Product Status 147
MEMS Nerve Ablation Device – Facial Glabellar Frowning – Product Description 147
Erchonia Corp Pipeline Products & Ongoing Clinical Trials Overview 148
Erchonia HLS Laser – Product Status 148
Erchonia HLS Laser – Product Description 148
Erchonia ML Scanner – Female Hair Restoration – Product Status 149
Erchonia ML Scanner – Female Hair Restoration – Product Description 149
Erchonia ML Scanner – Venous Stasis Ulcers – Product Status 149
Erchonia ML Scanner – Venous Stasis Ulcers – Product Description 150
Establishment Labs SA Pipeline Products & Ongoing Clinical Trials Overview 151
Mia Femtech – Product Status 152
Mia Femtech – Product Description 152
Motiva Ergonomix – Product Status 152
Motiva Ergonomix – Product Description 153
Motiva Ergonomix2 – Product Status 153
Motiva Ergonomix2 – Product Description 153
Motiva Ergonomix2 Diamond Breast Implant – Product Status 154
Motiva Ergonomix2 Diamond Breast Implant – Product Description 154
Motiva Flora Tissue Expander – Product Status 154
Motiva Flora Tissue Expander – Product Description 155
Motiva Implant Matrix SilkSurface PLUS With Q Inside Safety – Product Status 155
Motiva Implant Matrix SilkSurface PLUS With Q Inside Safety – Product Description 155
Motiva Implant Matrix VelvetSurface PLUS With Q Inside Safety – Product Status 156
Motiva Implant Matrix VelvetSurface PLUS With Q Inside Safety – Product Description 156
Motiva MIA – Product Status 156
Motiva MIA – Product Description 157
Motiva Round – Product Status 157
Motiva Round – Product Description 158
SmoothSilk Anatomical Tissue Expander – Product Status 158
SmoothSilk Anatomical Tissue Expander – Product Description 158
Establishment Labs SA – Ongoing Clinical Trials Overview 159
Motiva Round – Study of the Long-Term Effectiveness of the Motiva Implants Round and Round Ergonomix in Primary and Revision Breast Augmentation 160
Motiva Round – Study of the Safety and Effectiveness of the Motiva Implants Silicone Gel-filled Breast Implants SmoothSilk/SilkSurface in Subjects who are Undergoing Primary Breast Augmentation, Primary Breast Reconstruction and Revision Surgery 160
Motiva Implant Matrix SilkSurface PLUS With Q Inside Safety – Study of the Safety and Effectiveness of the Motiva Implants Silicone Gel-filled Breast Implants SmoothSilk/SilkSurface in Subjects who are Undergoing Primary Breast Augmentation, Primary Breast Reconstruction and Revision Surgery 161
Motiva Ergonomix – Study of the Long-Term Effectiveness of the Motiva Implants Round and Round Ergonomix in Primary and Revision Breast Augmentation 162
Motiva Ergonomix – Study of the Safety and Effectiveness of the Motiva Implants Silicone Gel-filled Breast Implants SmoothSilk/SilkSurface in Subjects who are Undergoing Primary Breast Augmentation, Primary Breast Reconstruction and Revision Surgery 162
SmoothSilk Anatomical Tissue Expander – Immunological Analysis of Capsular Tissue Formed Around Expanders With Varying Surface Topography in Women Undergoing Bilateral Nipple or Skin Sparing Mastectomy 163
SmoothSilk Anatomical Tissue Expander – Study of the Safety and Effectiveness of the Motiva Implants Silicone Gel-filled Breast Implants SmoothSilk/SilkSurface in Subjects who are Undergoing Primary Breast Augmentation, Primary Breast Reconstruction and Revision Surgery 163
Motiva Flora Tissue Expander – Post-Marketing Clinical Follow-Up Study to Confirm the Safety and Effectiveness/Performance of Motiva Flora Tissue Expander in Staged Breast Reconstruction Surgery 164
Motiva Flora Tissue Expander – Retrospective Data Collection on the Use of Motiva Flora TE in Breast Reconstruction 164
Motiva MIA – A Study to Evaluate the Motiva Mia System for Minimally Invasive Augmentation in Patients Undergoing Breast Implants 165
Motiva MIA – Multicenter Study to Evaluate Motiva Mia System for Minimally Invasive Augmentation in Thailand 165
Eternity Healthcare Inc Pipeline Products & Ongoing Clinical Trials Overview 166
Hand-held Laser Hair Removal Device – Product Status 166
Hand-held Laser Hair Removal Device – Product Description 166
EternoGen Aesthetics LLC Pipeline Products & Ongoing Clinical Trials Overview 167
Cellifique – Product Status 167
Cellifique – Product Description 167
Excita Medical Pipeline Products & Ongoing Clinical Trials Overview 168
Electrical Muscle Stimulation Device – Product Status 168
Electrical Muscle Stimulation Device – Product Description 168
Fillmed Laboratoires Pipeline Products & Ongoing Clinical Trials Overview 169
NCTF 135 – Product Status 169
NCTF 135 – Product Description 169
NCTF 135 HA – Product Status 170
NCTF 135 HA – Product Description 170
FixNip Ltd Pipeline Products & Ongoing Clinical Trials Overview 171
FixNip NRI – Product Status 171
FixNip NRI – Product Description 171
Florida International University Pipeline Products & Ongoing Clinical Trials Overview 172
Cosmetic Skin Treatment Device – Product Status 172
Cosmetic Skin Treatment Device – Product Description 172
Forticell Bioscience Inc Pipeline Products & Ongoing Clinical Trials Overview 173
Haptized Collagen Tissue Augmentation Device – Product Status 173
Haptized Collagen Tissue Augmentation Device – Product Description 173
Fourth State Medicine Ltd Pipeline Products & Ongoing Clinical Trials Overview 174
Nebulaskin – Product Status 174
Nebulaskin – Product Description 174
Fumailei Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 175
ForePico – Product Status 175
ForePico – Product Description 175
Long-Pulse Width Laser Therapy Instrument – Product Status 176
Long-Pulse Width Laser Therapy Instrument – Product Description 176
G2GBIO Inc Pipeline Products & Ongoing Clinical Trials Overview 177
GB-3001 – Product Status 177
GB-3001 – Product Description 177
Galderma SA Pipeline Products & Ongoing Clinical Trials Overview 178
Biostimulator Filler – Product Status 178
Biostimulator Filler – Product Description 178
GC Aesthetics Plc Pipeline Products & Ongoing Clinical Trials Overview 179
PERLE – Product Status 179
PERLE – Product Description 179
Genial Light Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 180
Genial Bone Laser – Product Status 180
Genial Bone Laser – Product Description 180
Genzyme Corp Pipeline Products & Ongoing Clinical Trials Overview 181
Prevelle Lift – Product Status 181
Prevelle Lift – Product Description 181
Giant Biogene Holding Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 182
Cross-Linking Recombinant Collagen Skin Rejuvenation Gel – Product Status 182
Cross-Linking Recombinant Collagen Skin Rejuvenation Gel – Product Description 182
Recombinant Collagen Skin Rejuvenation Gel – Product Status 183
Recombinant Collagen Skin Rejuvenation Gel – Product Description 183
Recombinant Collagen Sterile Dressing – Product Status 183
Recombinant Collagen Sterile Dressing – Product Description 184
Hallura Ltd Pipeline Products & Ongoing Clinical Trials Overview 185
BiOLinkMatrix Gel – Product Status 185
BiOLinkMatrix Gel – Product Description 185
Hyaluronic Acid Dermal Filler – Product Status 186
Hyaluronic Acid Dermal Filler – Product Description 186
HansBiomed Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 187
Bellagel SmoothFine – Product Status 187
Bellagel SmoothFine – Product Description 187
HansBiomed Co Ltd – Ongoing Clinical Trials Overview 188
Bellagel SmoothFine – Clinical Trial to Evaluate the Safety and Efficacy of Cohesive Silicone Gel-filled Breast Implant (CoSBI) in Women Aged 22 and Over with Breast Reconstruction or Augmentation Mammoplasty 189
Healshape SAS Pipeline Products & Ongoing Clinical Trials Overview 190
3D Printed Breast Implant – Product Status 190
3D Printed Breast Implant – Product Description 190
Histogen Inc Pipeline Products & Ongoing Clinical Trials Overview 191
HST002 – Product Status 191
HST002 – Product Description 191
Hugel Inc Pipeline Products & Ongoing Clinical Trials Overview 192
The Chaeum Pure No.1 – Product Status 192
The Chaeum Pure No.1 – Product Description 193
The Chaeum Pure No.2 – Product Status 193
The Chaeum Pure No.2 – Product Description 193
The Chaeum Pure No.3 – Product Status 194
The Chaeum Pure No.3 – Product Description 194
The Chaeum Pure No.4 – Product Status 194
The Chaeum Pure No.4 – Product Description 195
The Chaeum Style – Product Status 195
The Chaeum Style – Product Description 195
Ilooda Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 196
reepot Nd; YAG Laser System – Product Status 196
reepot Nd; YAG Laser System – Product Description 196
ImpLite Ltd. Pipeline Products & Ongoing Clinical Trials Overview 197
Freedom Implant – Product Status 197
Freedom Implant – Product Description 197
InMode Ltd Pipeline Products & Ongoing Clinical Trials Overview 198
Next Generation Evoke – Product Status 198
Next Generation Evoke – Product Description 198
Trim II – Product Status 199
Trim II – Product Description 199
InMode Ltd – Ongoing Clinical Trials Overview 200
Trim II – Safety and Efficacy of Trim II for Non-invasive Lipolysis and Circumference Reduction of Abdomen 201
Innova Medical Design Pipeline Products & Ongoing Clinical Trials Overview 202
Aesthetic Injection – Product Status 202
Aesthetic Injection – Product Description 202
Innovia LLC Pipeline Products & Ongoing Clinical Trials Overview 203
Breast Implant Shell – Product Status 203
Breast Implant Shell – Product Description 203
Wrinkle Filler – Product Status 204
Wrinkle Filler – Product Description 204
inSoma Bio Inc Pipeline Products & Ongoing Clinical Trials Overview 205
Fractomer – Product Status 205
Fractomer – Product Description 205
Intuitive Surgical Inc Pipeline Products & Ongoing Clinical Trials Overview 206
RF Laser Device – Product Status 206
RF Laser Device – Product Description 206
Inventage Lab Inc Pipeline Products & Ongoing Clinical Trials Overview 207
IVL-MD-B001 – Product Status 207
IVL-MD-B001 – Product Description 207
IVL-MD-F001 – Product Status 208
IVL-MD-F001 – Product Description 208
IVL-MD-F002 – Product Status 208
IVL-MD-F002 – Product Description 209
Jinwoo Bio Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 210
HA Filler – Product Status 210
HA Filler – Product Description 210
HA Thread Filler – Product Status 211
HA Thread Filler – Product Description 211
Wool Filler – Product Status 212
Wool Filler – Product Description 212
Jointechlabs Inc Pipeline Products & Ongoing Clinical Trials Overview 213
Mini-Stem System – Product Status 213
Mini-Stem System – Product Description 213
Juvenis Ltd Pipeline Products & Ongoing Clinical Trials Overview 214
Tenergel – Product Status 214
Tenergel – Product Description 214
Khorionyx SA Pipeline Products & Ongoing Clinical Trials Overview 215
Autologous Globin Dermal Filler – Product Status 215
Autologous Globin Dermal Filler – Product Description 215
Laboratoires Vivacy SAS Pipeline Products & Ongoing Clinical Trials Overview 216
IPN-21-SENSE Dermal Filler – Product Status 216
IPN-21-SENSE Dermal Filler – Product Description 216
Laboratoires Vivacy SAS – Ongoing Clinical Trials Overview 217
IPN-21-SENSE Dermal Filler – A Multicenter, Prospective, Randomized Controlled Clinical Study of IPN-21-SENSE, a Novel Hyaluronic Acid Based Gel for Volume Deficiency in the Mid-face 218
Lattice Medical Pipeline Products & Ongoing Clinical Trials Overview 219
MATTISSE – Product Status 219
MATTISSE – Product Description 219
Lattice Medical – Ongoing Clinical Trials Overview 220
MATTISSE – First-in-human, Study of MATTISSE Tissue Engineering Chamber in Adult Female Patients Undergoing Immediate Breast Reconstruction After Mastectomy for Cancer 221
Lavish Pipeline Products & Ongoing Clinical Trials Overview 222
Straberi Epistamp Microneedling Device – Product Status 222
Straberi Epistamp Microneedling Device – Product Description 222
Lavish – Ongoing Clinical Trials Overview 223
Straberi Epistamp Microneedling Device – Clinical Trial Study for the Use of Straberi Epistamp Needling Device to Treat Postinflammatory Hyperpigmentation (PIH) 224
Straberi Epistamp Microneedling Device – Clinical Trial Study for the Use of Straberi Microneedling Device to Treat Atrophic Acne Scarring 224
Straberi Epistamp Microneedling Device – Face Study of the Effects of Needling Using the Straberi Epistamp for the Improvement of Fine Lines and Skin Laxity. 224
LG Chem Ltd Pipeline Products & Ongoing Clinical Trials Overview 225
Dermal Filler (LR19059) – Product Status 225
Dermal Filler (LR19059) – Product Description 225
Dermal Filler (LR19093) – Product Status 226
Dermal Filler (LR19093) – Product Description 226
Hyaluronic Acid Filler (LR19094) – Product Status 226
Hyaluronic Acid Filler (LR19094) – Product Description 226
Hyaluronic Acid Filler (LR20024) – Product Status 227
Hyaluronic Acid Filler (LR20024) – Product Description 227
Next Generation Hyaluronic Acid Filler (LR20008) – Product Status 227
Next Generation Hyaluronic Acid Filler (LR20008) – Product Description 227
YVOIRE Y-Solution 360 – Product Status 228
YVOIRE Y-Solution 360 – Product Description 228
YVOIRE Y-Solution 540 – Product Status 228
YVOIRE Y-Solution 540 – Product Description 229
YVOIRE Y-Solution 720 – Product Status 229
YVOIRE Y-Solution 720 – Product Description 229
LG Chem Ltd – Ongoing Clinical Trials Overview 230
YVOIRE Y-Solution 360 – A Multicenter, Randomized, Rater-Blinded, No-Treatment Control Design Clinical Investigation to Evaluate the Effectiveness and Safety of YVOIRE Y-Solution 360 for Lip Augmentation 231
YVOIRE Y-Solution 540 – A Randomized, Multicenter, Evaluator-blinded, Active-controlled, Parallel-group Design Investigation to Evaluate the Effectiveness and Safety of YVOIRE Y-Solution 540 Versus YVOIRE Volume Plus in Nasolabial Folds Injection 232
YVOIRE Y-Solution 720 – A Multicenter, Randomized, Rater-blinded, No-treatment Control Design Clinical Study to Evaluate the Effectiveness and Safety of YVOIRE Y-Solution 720 Injected Into the Mid-face 233
LifeCell Corp Pipeline Products & Ongoing Clinical Trials Overview 234
Artia – Breast Reconstruction – Product Status 234
Artia – Breast Reconstruction – Product Description 234
Strattice Reconstructive Tissue Matrix – Face Lift – Product Status 235
Strattice Reconstructive Tissue Matrix – Face Lift – Product Description 235
LifeSprout Inc Pipeline Products & Ongoing Clinical Trials Overview 236
Injectable Nanofiber-Hydrogel Matrix – Product Status 236
Injectable Nanofiber-Hydrogel Matrix – Product Description 236
Lumina – Product Status 237
Lumina – Product Description 237
LifeSprout Inc – Ongoing Clinical Trials Overview 238
Lumina – A Prospective, Randomized, Controlled, Multi-center Study of the Safety and Effectiveness of Lumina in the Treatment of Nasolabial Folds 239
LigthSense Israel Ltd Pipeline Products & Ongoing Clinical Trials Overview 240
S2 Picosecond Laser – Product Status 240
S2 Picosecond Laser – Product Description 240
LigthSense Israel Ltd – Ongoing Clinical Trials Overview 241
S2 Picosecond Laser – A Single-center, Open-label, Uncontrolled Pilot Study to Evaluate the Safety and Efficacy of S2 – Multi-wavelength Laser for Tattoo Removal 242
ManaMed Inc Pipeline Products & Ongoing Clinical Trials Overview 243
FB Professional LED Red Light Therapy System – Product Status 243
FB Professional LED Red Light Therapy System – Product Description 243
Medality Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 244
HydraSolve T2D – Product Status 244
HydraSolve T2D – Product Description 244
Medality Medical LLC – Ongoing Clinical Trials Overview 245
HydraSolve T2D – Evaluation of the Removal of Excess Intra-abdominal Fat in Subjects with Type 2 Diabetes and Obesity, Using the HydraSolve T2D System, on Glucose Control, Insulin Resistance and Body Weight 246
Medifirst Solutions Inc Pipeline Products & Ongoing Clinical Trials Overview 247
Green Laser – 532 nm – Product Status 247
Green Laser – 532 nm – Product Description 247
Medytox Inc Pipeline Products & Ongoing Clinical Trials Overview 248
MT943 – Product Status 248
MT943 – Product Description 248
MT945 – Product Status 249
MT945 – Product Description 249
MTP50 – Product Status 249
MTP50 – Product Description 249
MTP51 – Product Status 250
MTP51 – Product Description 250
Neuramis Deep Lidocaine – Product Status 250
Neuramis Deep Lidocaine – Product Description 251
Neuramis Volume Lidocaine – Product Status 251
Neuramis Volume Lidocaine – Product Description 251
Mentor Worldwide LLC Pipeline Products & Ongoing Clinical Trials Overview 252
Mentor – Becker Round Adjustable Gel Implant – Product Status 252
Mentor – Becker Round Adjustable Gel Implant – Product Description 252
Merz North America Inc Pipeline Products & Ongoing Clinical Trials Overview 253
VP1 Lido US – Product Status 253
VP1 Lido US – Product Description 253
Merz North America Inc – Ongoing Clinical Trials Overview 254
VP1 Lido US – A Prospective, Multicenter, Randomized, Comparator-controlled, Evaluator-blinded Study to Evaluate the Safety and Effectiveness of VP1 Lido US for Volume Augmentation of the Cheek 255
Merz Pharma GmbH & Co KgaA Pipeline Products & Ongoing Clinical Trials Overview 256
![]()