Airway Management – Pipeline Products by Stage of Development 22
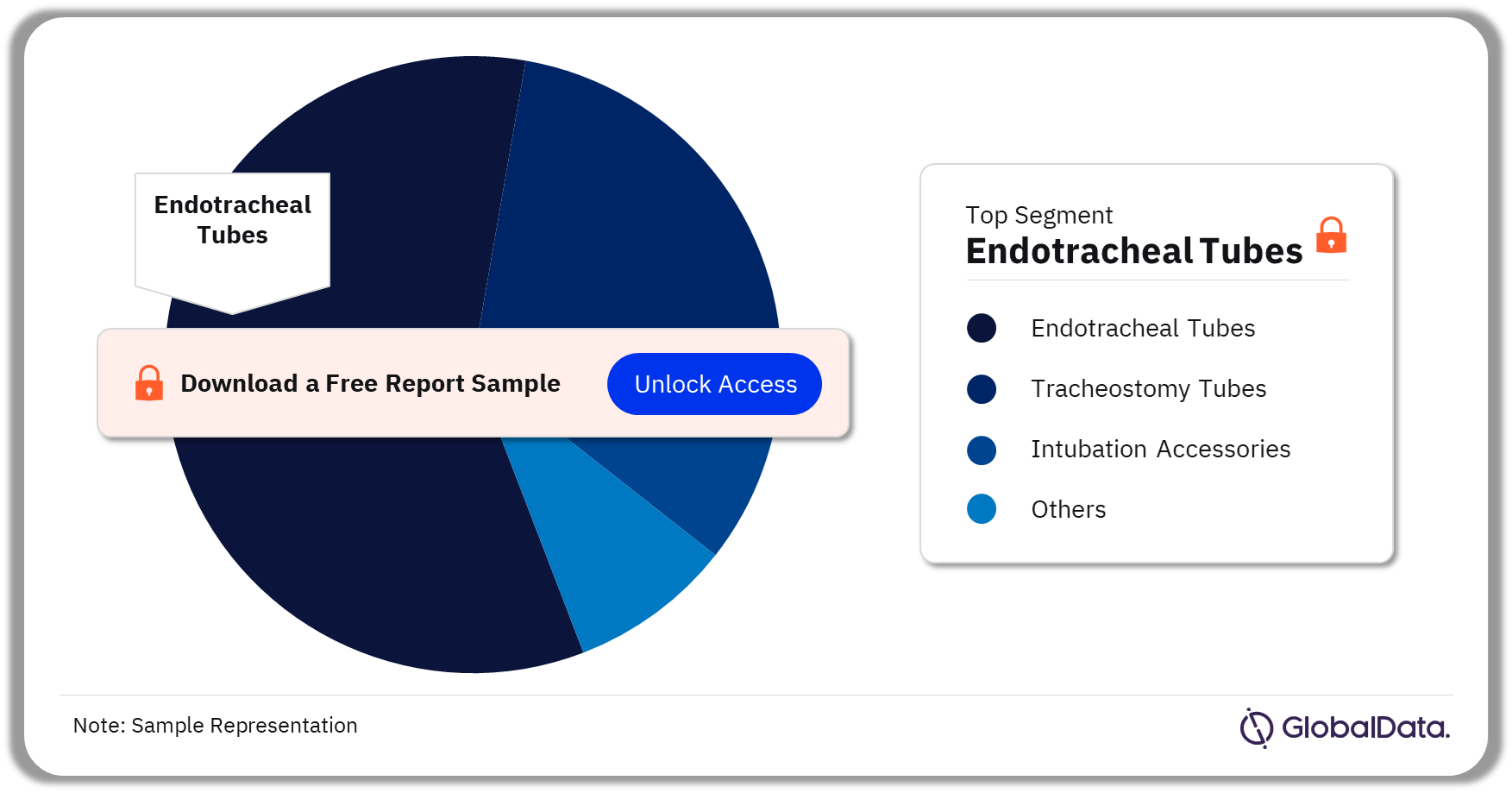
Airway Management – Pipeline Products by Segment 23
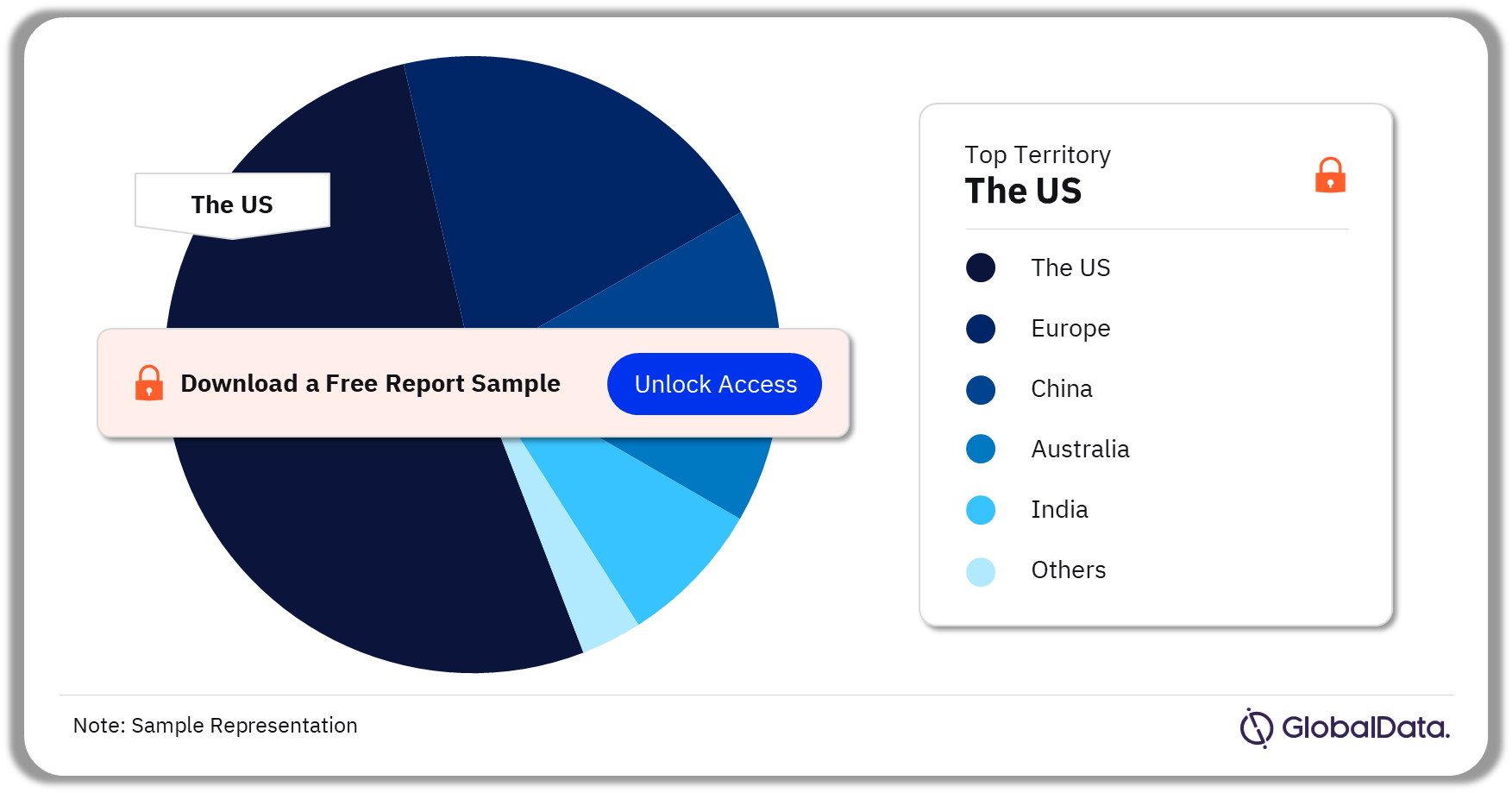
Airway Management – Pipeline Products by Territory 24
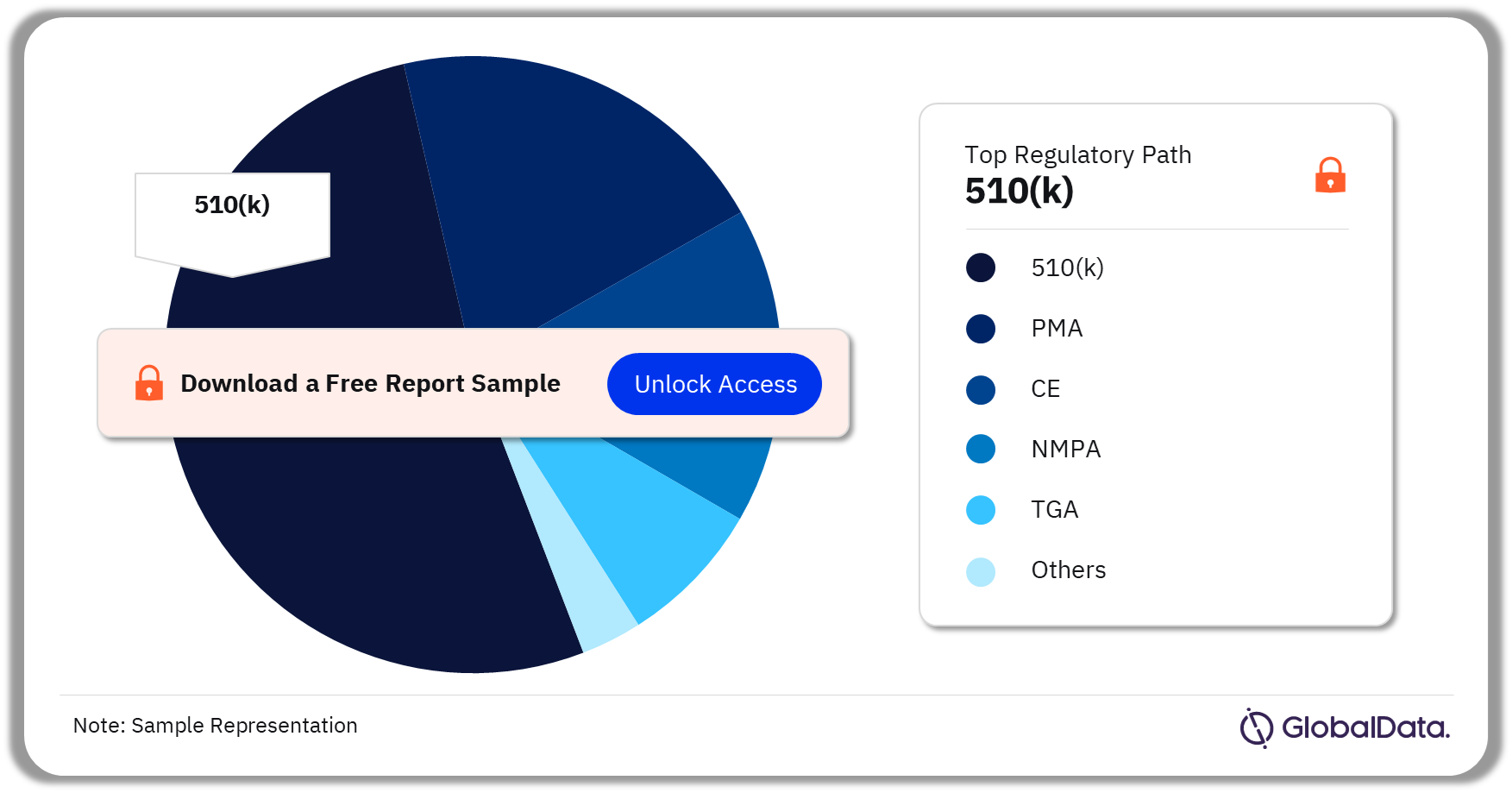
Airway Management – Pipeline Products by Regulatory Path 25
Airway Management – Pipeline Products by Estimated Approval Date 26
Airway Management – Ongoing Clinical Trials 27
Airway Management Companies – Pipeline Products by Stage of Development 28
Airway Management – Pipeline Products by Stage of Development 33
Actuated Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 37
Endotracheal Clearing Device – Product Status 37
Endotracheal Clearing Device – Product Description 37
Neo ET-Clear – Product Status 38
Neo ET-Clear – Product Description 38
ADM Tronics Unlimited Inc Pipeline Products & Ongoing Clinical Trials Overview 39
LSI System – Product Status 39
LSI System – Product Description 39
Adroit USA, Inc Pipeline Products & Ongoing Clinical Trials Overview 40
Battery Powered Laryngoscope – Product Status 40
Battery Powered Laryngoscope – Product Description 40
Airway Innovations LLC Pipeline Products & Ongoing Clinical Trials Overview 41
Air Aid Endotracheal Tube Holder – Product Status 41
Air Aid Endotracheal Tube Holder – Product Description 41
All India Institute of Medical Sciences Pipeline Products & Ongoing Clinical Trials Overview 42
Endotracheal Intubation System – Product Status 42
Endotracheal Intubation System – Product Description 42
Suctioning Machine – Tracheostomy Tubes – Product Status 43
Suctioning Machine – Tracheostomy Tubes – Product Description 43
Allvivo Vascular Inc Pipeline Products & Ongoing Clinical Trials Overview 44
Antimicrobial Endotracheal Tube – Product Status 44
Antimicrobial Endotracheal Tube – Product Description 44
Avir Medical Pte Ltd Pipeline Products & Ongoing Clinical Trials Overview 45
Endotracheal Tube – Pneumonia – Product Status 45
Endotracheal Tube – Pneumonia – Product Description 45
Baylor College of Medicine Pipeline Products & Ongoing Clinical Trials Overview 46
Anti-Infective Endotracheal Tube – Product Status 46
Anti-Infective Endotracheal Tube – Product Description 46
BB Medical Surgical Inc Pipeline Products & Ongoing Clinical Trials Overview 47
SAAVI Device – Product Status 47
SAAVI Device – Product Description 47
Ben-Gurion University of the Negev Pipeline Products & Ongoing Clinical Trials Overview 48
Aeroself – Product Status 48
Aeroself – Product Description 48
Mucus Elimination Device – Product Status 49
Mucus Elimination Device – Product Description 49
Biovo Technologies Ltd Pipeline Products & Ongoing Clinical Trials Overview 50
HyperFlex-LM – Product Status 50
HyperFlex-LM – Product Description 50
Bleep LLC Pipeline Products & Ongoing Clinical Trials Overview 51
DreamPort – Eclipse – Product Status 51
DreamPort – Eclipse – Product Description 51
Bleep LLC – Ongoing Clinical Trials Overview 52
DreamPort – Eclipse – Randomized Controlled Trial to Compare Effectiveness of DreamPort-Eclipse to a Traditional Nasal Mask 53
Brio Device, LLC Pipeline Products & Ongoing Clinical Trials Overview 54
Neonatal-Sized Articulating Video Stylet – Product Status 54
Neonatal-Sized Articulating Video Stylet – Product Description 54
Cedars-Sinai Medical Center Pipeline Products & Ongoing Clinical Trials Overview 55
Oxygenating Bite Block – Product Status 55
Oxygenating Bite Block – Product Description 55
Cognita Labs LLC Pipeline Products & Ongoing Clinical Trials Overview 56
SonoHeal – Product Status 56
SonoHeal – Product Description 56
Columbia University Pipeline Products & Ongoing Clinical Trials Overview 57
Endotracheal Intubation Device – Product Status 57
Endotracheal Intubation Device – Product Description 57
Cryofocus Medtech Shanghai Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 58
Asthma Cryoablation System – Product Status 58
Asthma Cryoablation System – Product Description 58
COPD Cryospray System – Product Status 59
COPD Cryospray System – Product Description 59
Tuberculosis Cryospray System – Product Status 59
Tuberculosis Cryospray System – Product Description 60
Daiichi Medical Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 61
Artificial Airway Device – Product Status 61
Artificial Airway Device – Product Description 61
DMF Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 62
Memsorb – Product Status 62
Memsorb – Product Description 62
DMF Medical Inc – Ongoing Clinical Trials Overview 63
Memsorb – A New and Innovative Method for CO2 Removal in Anesthetic Circuits 64
Memsorb – Decreasing Environmental Impact and Costs of Using Inhalational Anesthetics by Replacing Chemical Absorbers with an Innovative Carbon Dioxide Membrane Filter System – a Prospective, Randomized, Clinical Trial 64
Down From The Door LLC Pipeline Products & Ongoing Clinical Trials Overview 65
Airway Stent – Product Status 65
Airway Stent – Product Description 65
Drexel University Pipeline Products & Ongoing Clinical Trials Overview 66
Endotracheal Tube Curve Stabilizer – Product Status 66
Endotracheal Tube Curve Stabilizer – Product Description 66
Ultrasound Guided Insertion Tube Monitor Device – Product Status 67
Ultrasound Guided Insertion Tube Monitor Device – Product Description 67
Emory University Pipeline Products & Ongoing Clinical Trials Overview 68
Pop Up Tent System – Product Status 68
Pop Up Tent System – Product Description 68
Enox Biopharma Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 69
Endotracheal Tube – Product Status 69
Endotracheal Tube – Product Description 69
Fogless International AB Pipeline Products & Ongoing Clinical Trials Overview 70
Endotracheal Tube – Product Status 70
Endotracheal Tube – Product Description 70
Free Flow Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 71
Free Flow Medical Coil System – Product Status 71
Free Flow Medical Coil System – Product Description 71
Gala Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 72
RheOx System – Product Status 72
RheOx System – Product Description 72
Gala Therapeutics Inc – Ongoing Clinical Trials Overview 73
RheOx System – A Clinical Evaluation of the RheOx Bronchial Rheoplasty System for the Treatment of the Symptoms of Chronic Bronchitis in Adult Patients with COPD 74
RheOx System – A Clinical Evaluation of the RheOx Bronchial Rheoplasty System for the Treatment of the Symptoms of Chronic Bronchitis in Chinese Adult Patients With COPD 74
RheOx System – A Feasibility Study: A Safety Evaluation of RheOx on Patients with Chronic Bronchitis in the United States 74
RheOx System – RheOx European Post-market Clinical Study 75
H. Lee Moffitt Cancer Center & Research Institute Inc Pipeline Products & Ongoing Clinical Trials Overview 76
Improved Stylet – Endotracheal Tube Placement – Product Status 76
Improved Stylet – Endotracheal Tube Placement – Product Description 76
Hannover Medical School Pipeline Products & Ongoing Clinical Trials Overview 77
I-Scoop – Product Status 77
I-Scoop – Product Description 77
Iasis Molecular Sciences Inc Pipeline Products & Ongoing Clinical Trials Overview 78
SupraGuard ETT – Product Status 78
SupraGuard ETT – Product Description 78
Illumitube Pipeline Products & Ongoing Clinical Trials Overview 79
Intubation Tube – Product Status 79
Intubation Tube – Product Description 79
ImThera Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 80
LivaNova aura6000 System – Product Status 80
LivaNova aura6000 System – Product Description 80
ImThera Medical Inc – Ongoing Clinical Trials Overview 81
LivaNova aura6000 System – Treating Obstructive Sleep Apnea Using Targeted Hypoglossal Neurostimulation 82
Indian Institute of Technology Guwahati Pipeline Products & Ongoing Clinical Trials Overview 83
Aerosol Obstruction Box – Product Status 83
Aerosol Obstruction Box – Product Description 83
Innovations Unlimited LLC Pipeline Products & Ongoing Clinical Trials Overview 84
TrachAlarm – Product Status 84
TrachAlarm – Product Description 84
Innovent Medical Solutions Ltd Pipeline Products & Ongoing Clinical Trials Overview 85
Home Care Respiratory Clearance System – Product Status 85
Home Care Respiratory Clearance System – Product Description 85
Second Generation Device – Product Status 86
Second Generation Device – Product Description 86
Innovex Medical Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 87
Respiratory Occlusion Balloon Catheter – Product Status 87
Respiratory Occlusion Balloon Catheter – Product Description 87
Inspire Medical Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 88
Inspire VI – Product Status 88
Inspire VI – Product Description 88
Inspire VII – Product Status 89
Inspire VII – Product Description 89
Inspire5 Device – Product Status 89
Inspire5 Device – Product Description 90
Intumed Ltd. Pipeline Products & Ongoing Clinical Trials Overview 91
ALMA100 – Product Status 91
ALMA100 – Product Description 91
Johns Hopkins University Pipeline Products & Ongoing Clinical Trials Overview 92
Drug Eluting Airway Stent – Product Status 92
Drug Eluting Airway Stent – Product Description 92
Spiral Slit Endotracheal Tube – Product Status 93
Spiral Slit Endotracheal Tube – Product Description 93
Tracheoesophageal Prosthesis Insufflator – Product Status 93
Tracheoesophageal Prosthesis Insufflator – Product Description 94
Johns Hopkins University School of Medicine Pipeline Products & Ongoing Clinical Trials Overview 95
CricSpike – Product Status 95
CricSpike – Product Description 95
Kaohsiung Medical University Pipeline Products & Ongoing Clinical Trials Overview 96
Bougie – Product Status 96
Bougie – Product Description 96
KK Women’s and Children’s Hospital Pipeline Products & Ongoing Clinical Trials Overview 97
Force Sensor Device – Product Status 97
Force Sensor Device – Product Description 97
Laval University Pipeline Products & Ongoing Clinical Trials Overview 98
Right Double Lumen Endotracheal Tube – Product Status 98
Right Double Lumen Endotracheal Tube – Product Description 98
LifeServe Innovations, LLC Pipeline Products & Ongoing Clinical Trials Overview 99
Cobra Tracheostomy Device – Product Status 99
Cobra Tracheostomy Device – Product Description 99
LifeTech Respiratory Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 100
Cinenses Lung Volume Reduction Reverser System – Product Status 100
Cinenses Lung Volume Reduction Reverser System – Product Description 100
LifeTech Respiratory Technology Co Ltd – Ongoing Clinical Trials Overview 101
Cinenses Lung Volume Reduction Reverser System – Evaluation of the Cinenses Lung Volume Reduction Reverser System in Treating Patients with Severe Emphysema 102
Cinenses Lung Volume Reduction Reverser System – Prospective, Multicenter, Randomized Controlled Study to Evaluate Safety and Efficacy of the Cinenses Tm Lung Volume Reduction Reverser System in Treating Patients with Severe Emphysema 102
Lingua Flex LLC Pipeline Products & Ongoing Clinical Trials Overview 103
Linguaflex Tongue Retractor – Product Status 103
Linguaflex Tongue Retractor – Product Description 103
Lingua Flex LLC – Ongoing Clinical Trials Overview 104
Linguaflex Tongue Retractor – Non-Randomized, Multi-Site, Single-Arm Study of the LinguaFlex Tongue Retractor (LTR) for the Treatment of Moderate to Severe Obstructive Sleep Apnea and Snoring in Adult Subjects 105
LMA Optimizer BV Pipeline Products & Ongoing Clinical Trials Overview 106
LarynxLock – Product Status 106
LarynxLock – Product Description 106
Lundquist Pipeline Products & Ongoing Clinical Trials Overview 107
Airway Management Device – Product Status 107
Airway Management Device – Product Description 107
Endotrachael Intubation Device – Product Status 108
Endotrachael Intubation Device – Product Description 108
Magnets In Me Inc Pipeline Products & Ongoing Clinical Trials Overview 109
Magnap – Product Status 109
Magnap – Product Description 109
Magnets In Me Inc – Ongoing Clinical Trials Overview 110
Magnap – Magnetic Apnea Prevention (MAGNAP) Device to Treat Obstructive Sleep Apnea: First-in-human Study of Feasibility and Safety 111
Maisonneuve-Rosemont Hospital Pipeline Products & Ongoing Clinical Trials Overview 112
VACCIN Box – Product Status 112
VACCIN Box – Product Description 112
Maisonneuve-Rosemont Hospital – Ongoing Clinical Trials Overview 113
VACCIN Box – First Randomized Controlled Clinical Evaluation of the Impact of Anti-aerosol VACCIN Box on the Videolaryngoscopic Intubation Procedure 114
Materialise NV Pipeline Products & Ongoing Clinical Trials Overview 115
3-D Printed Splint – Product Status 115
3-D Printed Splint – Product Description 115
Medical Device Investments, Inc. Pipeline Products & Ongoing Clinical Trials Overview 116
Anesthesia Intra-Oral Monitoring System – Product Status 116
Anesthesia Intra-Oral Monitoring System – Product Description 116
Endotracheal Extubation Safe Tube – Product Status 117
Endotracheal Extubation Safe Tube – Product Description 117
Orogastric Placement Intra-Oral Monitoring System – Product Status 117
Orogastric Placement Intra-Oral Monitoring System – Product Description 118
Miach Medical Innovations Pipeline Products & Ongoing Clinical Trials Overview 119
SMART-Tube – Product Status 119
SMART-Tube – Product Description 119
Minnesota Health Solutions Corporation Pipeline Products & Ongoing Clinical Trials Overview 120
Home Pulmonary Rehabilitation System – Product Status 120
Home Pulmonary Rehabilitation System – Product Description 120
Neonatal/Pediatric Securement System – Product Status 121
Neonatal/Pediatric Securement System – Product Description 121
N8 Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 122
CeraShield Coated Endotracheal Tube – Product Status 122
CeraShield Coated Endotracheal Tube – Product Description 122
N8 Medical LLC – Ongoing Clinical Trials Overview 123
CeraShield Coated Endotracheal Tube – Ceragenin Coated Endotracheal Tubes for the Eradication of Ventilator Associated Pneumonia – A Prospective, Longitudinal, Cross-over, Interrupted Time, Implementation Study (CEASE VAP Study) 124
CeraShield Coated Endotracheal Tube – CeraShiel Endotracheal Tube Feasibility Study 124
CeraShield Coated Endotracheal Tube – Clinical Study to Evaluate CeraShield Coated Endotracheal Tubes 124
Nanofiber Solutions LLC Pipeline Products & Ongoing Clinical Trials Overview 125
Off-The-Shelf Trachea Patch – Product Status 125
Off-The-Shelf Trachea Patch – Product Description 125
NanoVibronix Inc Pipeline Products & Ongoing Clinical Trials Overview 126
TrachShield – Product Status 126
TrachShield – Product Description 126
National University of Singapore Pipeline Products & Ongoing Clinical Trials Overview 127
WIND – Product Status 127
WIND – Product Description 127
Naval Medical Research Center Pipeline Products & Ongoing Clinical Trials Overview 128
Endotracheal Tube Stabilization Device – Product Status 128
Endotracheal Tube Stabilization Device – Product Description 128
Nevap, Inc Pipeline Products & Ongoing Clinical Trials Overview 129
Ventilator Tube – Product Status 129
Ventilator Tube – Product Description 129
Ningbo Senscure Biotechnology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 130
Airway Cryotherapy System – Product Status 130
Airway Cryotherapy System – Product Description 130
Ningbo Senscure Biotechnology Co Ltd – Ongoing Clinical Trials Overview 131
Airway Cryotherapy System – A Prospective, Multi-center, Randomized Controlled Study to Evaluate the Safety and Effectiveness of Airway Cryotherapy System Using Non-inferiority Method 132
Airway Cryotherapy System – A Prospective, Multicenter, Randomized, Blinded, Parallel-controlled Clinical Trial on the Efficacy and Safety of Bronchoscopic Cryoablation Denervation with Airway Cryotherapy System for Treatment of Severe Asthma in Adults 132
Novlead Biotechnology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 133
Inofree Ultrasound Nasal Soothing Device – Product Status 133
Inofree Ultrasound Nasal Soothing Device – Product Description 133
Nyxoah SA Pipeline Products & Ongoing Clinical Trials Overview 134
Genio 2.1 – Product Status 134
Genio 2.1 – Product Description 135
Genio System – Product Status 135
Genio System – Product Description 135
Nyxoah SA – Ongoing Clinical Trials Overview 136
Genio System – A Multicenter Study to Assess the Safety and Effectiveness of the Genio Dual-sided Hypoglossal Nerve Stimulation System for the Treatment of Obstructive Sleep Apnea in Adults Subjects 137
Genio System – A Multicenter Study to Assess the Safety and Effectiveness of the Genio Dual-sided Hypoglossal Nerve Stimulation System for the Treatment of Obstructive Sleep Apnea in Subjects With Complete Concentric Collapse of the Soft Palate 137
Genio System – A Multicentre, Prospective, Open-label, 2 Groups Study to Assess the Safety and Performance of the Genio Bilateral Hypoglossal Nerve Stimulation System for the Treatment of Obstructive Sleep Apnoea in Adult Patients with and without Complete Concentric Collapse of the Soft Palate 137
Genio System – A Post-market Clinical Follow up of the Genio System for the Treatment of Obstructive Sleep Apnea in Adults 138
Genio 2.1 – A Multicenter Study to Assess the Safety and Effectiveness of the Genio Dual-sided Hypoglossal Nerve Stimulation System for the Treatment of Obstructive Sleep Apnea in Adults Subjects 139
Olifant Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 140
Tusk Tracheal Cannulator – Product Status 140
Tusk Tracheal Cannulator – Product Description 140
Oventus Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 141
Exvent PEEP Valve – Product Status 141
Exvent PEEP Valve – Product Description 141
O2Vent Connect – Product Status 142
O2Vent Connect – Product Description 142
O2Vent ONEPAP – Product Status 142
O2Vent ONEPAP – Product Description 143
O2Vent Plus – Product Status 143
O2Vent Plus – Product Description 143
Pneumocool LLC Pipeline Products & Ongoing Clinical Trials Overview 144
PneumoCool – Product Status 144
PneumoCool – Product Description 144
Pneumocool LLC – Ongoing Clinical Trials Overview 145
PneumoCool – Breathlessness Relieved by Employing Medical Air to Be Titrated by Hospitalized Patients to Improve Inpatient Experience 146
ProSomnus Sleep Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 147
ProSomnus EVO Sleep And Snore Device – Severe Obstructive Sleep Apnea – Product Status 147
ProSomnus EVO Sleep And Snore Device – Severe Obstructive Sleep Apnea – Product Description 147
ProSomnus Sleep Technologies Inc – Ongoing Clinical Trials Overview 148
ProSomnus EVO Sleep And Snore Device – Severe Obstructive Sleep Apnea – Comparison of First Line Non-invasive Treatment Options in Patients Diagnosed with Obstructive Sleep Apnea 149
RegenaGraft Pipeline Products & Ongoing Clinical Trials Overview 150
RegenaTrach – Product Status 150
RegenaTrach – Product Description 150
Respiosa BV Pipeline Products & Ongoing Clinical Trials Overview 151
Respiosa Implant System – Product Status 151
Respiosa Implant System – Product Description 151
Resultados y Calidad del Sistema Sanitario Publico de Andalucia Pipeline Products & Ongoing Clinical Trials Overview 152
Endotracheal Tube – Product Status 152
Endotracheal Tube – Product Description 152
Rutgers The State University of New Jersey Pipeline Products & Ongoing Clinical Trials Overview 153
Endotracheal Tube Placement Device – Product Status 153
Endotracheal Tube Placement Device – Product Description 153
Sanovas Inc Pipeline Products & Ongoing Clinical Trials Overview 154
Bronchial Thermoplasty Tool – Product Status 154
Bronchial Thermoplasty Tool – Product Description 154
Securisyn Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 155
SolidAIRity Sentry CT – Product Status 155
SolidAIRity Sentry CT – Product Description 155
Sedana Medical AB Pipeline Products & Ongoing Clinical Trials Overview 156
Sedaconda ACD – Product Status 156
Sedaconda ACD – Product Description 156
Sedana Medical AB – Ongoing Clinical Trials Overview 157
Sedaconda ACD – Comparison of an Inhaled Sedation Strategy to an Intravenous Sedation Strategy in Intensive Care Unit Patients Treated with Invasive Mechanical Ventilation : INASED Study 158
Sharklet Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 159
Sharklet Endotracheal Tube – Product Status 159
Sharklet Endotracheal Tube – Product Description 159
Silmag SA Pipeline Products & Ongoing Clinical Trials Overview 160
Mucus Suction Tube – Product Status 160
Mucus Suction Tube – Product Description 160
Tracheostomy Tube – Pediatric – Product Status 161
Tracheostomy Tube – Pediatric – Product Description 161
Sommetrics, Inc. Pipeline Products & Ongoing Clinical Trials Overview 162
aerFree – COVID-19 – Product Status 162
aerFree – COVID-19 – Product Description 162
aerSleep II – COVID-19 – Product Status 163
aerSleep II – COVID-19 – Product Description 163
aerSleep II – Sleep Apnea – Product Status 163
aerSleep II – Sleep Apnea – Product Description 164
aersleep System – Product Status 164
aersleep System – Product Description 164
cNEP Device – Snoring – Product Status 165
cNEP Device – Snoring – Product Description 165
Sommetrics, Inc. – Ongoing Clinical Trials Overview 166
aerSleep II – Sleep Apnea – Continuation Protocol for Study Using Negative Pressure to Reduce Apnea Trial (CO-STAR) 167
aerSleep II – Sleep Apnea – Study Using Negative Pressure to Reduce Apnea (SUPRA) 167
Somne LLC Pipeline Products & Ongoing Clinical Trials Overview 168
vNEP (Variable Negative External Pressure) Device – Product Status 168
vNEP (Variable Negative External Pressure) Device – Product Description 168
Sonoma Pharmaceuticals Inc Pipeline Products & Ongoing Clinical Trials Overview 169
Endotracheal Tube Device – Product Status 169
Endotracheal Tube Device – Product Description 169
Sree Chitra Tirunal Institute for Medical Sciences & Technology Pipeline Products & Ongoing Clinical Trials Overview 170
Universal Airway Device – Product Status 170
Universal Airway Device – Product Description 170
Stevens Institute of Technology Pipeline Products & Ongoing Clinical Trials Overview 171
Trachalert – Product Status 171
Trachalert – Product Description 171
Suzhou Xinmai Medical Equipment Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 172
SyMap Bronchial Ablation System – Product Status 172
SyMap Bronchial Ablation System – Product Description 172
Swiftsure Innovations Pipeline Products & Ongoing Clinical Trials Overview 173
SwishKit – Product Status 173
SwishKit – Product Description 173
Synchrony Medical Pipeline Products & Ongoing Clinical Trials Overview 174
LibAirty – Product Status 174
LibAirty – Product Description 174
Synchrony Medical – Ongoing Clinical Trials Overview 175
LibAirty – A Single center, Randomized-controlled, Crossover Clinical Study of LibAirty Airway Clearance System 176
LibAirty – Study to Compare the Effectiveness of Airway Clearance by LibAirty with the Standard of Care Solutions 176
Teleflex Inc Pipeline Products & Ongoing Clinical Trials Overview 177
Anti-Biofilm Endotracheal Tube – Product Status 177
Anti-Biofilm Endotracheal Tube – Product Description 177
TrachFlex Plus MRI with Suction – Product Status 178
TrachFlex Plus MRI with Suction – Product Description 178
The Chaim Sheba Medical Center Pipeline Products & Ongoing Clinical Trials Overview 179
Endotracheal Tube Securing Device – Product Status 179
Endotracheal Tube Securing Device – Product Description 179
The Chinese University of Hong Kong Pipeline Products & Ongoing Clinical Trials Overview 180
Endotracheal Tube – Product Status 180
Endotracheal Tube – Product Description 180
The University of British Columbia Pipeline Products & Ongoing Clinical Trials Overview 181
COVID-19 Barrier Box – Product Status 181
COVID-19 Barrier Box – Product Description 181
Tufts University Pipeline Products & Ongoing Clinical Trials Overview 182
Fiber Optic Intubating Device – Product Status 182
Fiber Optic Intubating Device – Product Description 182
Tutti Toot Ltd Pipeline Products & Ongoing Clinical Trials Overview 183
Tutti Toot Trumpet – Product Status 183
Tutti Toot Trumpet – Product Description 183
U.S. Department of Defence Pipeline Products & Ongoing Clinical Trials Overview 184
Hyperspectral Imaging Device – Product Status 184
Hyperspectral Imaging Device – Product Description 184
Uniformed Services University of the Health Sciences Pipeline Products & Ongoing Clinical Trials Overview 185
STEAM Device – Product Status 185
STEAM Device – Product Description 185
Universitas Padjadjaran Pipeline Products & Ongoing Clinical Trials Overview 186
XVent XMV 20 Frontliner – Product Status 186
XVent XMV 20 Frontliner – Product Description 186
University of California Irvine Pipeline Products & Ongoing Clinical Trials Overview 187
Disposable Medical Collar – Product Status 187
Disposable Medical Collar – Product Description 187
Microstimulator – Product Status 188
Microstimulator – Product Description 188
University of Colorado Pipeline Products & Ongoing Clinical Trials Overview 189
Difficult Airway Tube – Product Status 189
Difficult Airway Tube – Product Description 189
University of Florida Pipeline Products & Ongoing Clinical Trials Overview 190
Tracheal Punch Device – Product Status 190
Tracheal Punch Device – Product Description 190
University of Illinois at Chicago Pipeline Products & Ongoing Clinical Trials Overview 191
Endotracheal Tube – Product Status 191
Endotracheal Tube – Product Description 191
University of Michigan Pipeline Products & Ongoing Clinical Trials Overview 192
Airway Cradle – Product Status 192
Airway Cradle – Product Description 192
Endotracheal Solution Device – Product Status 193
Endotracheal Solution Device – Product Description 193
Nasal Congestion Device – Product Status 193
Nasal Congestion Device – Product Description 193
Pediatric Intubation Device – Product Status 194
Pediatric Intubation Device – Product Description 194
Self-Supporting Nasopharyngeal Airway Device – Product Status 194
Self-Supporting Nasopharyngeal Airway Device – Product Description 194
University of Michigan – Ongoing Clinical Trials Overview 195
Self-Supporting Nasopharyngeal Airway Device – Self-Supporting Nasopharyngeal Airway (ssNPA) Treating Upper Airway Obstruction in Hypotonia 196
University of Michigan Pediatric Device Consortium Pipeline Products & Ongoing Clinical Trials Overview 197
Airflow Acoustic Sensor – Product Status 197
Airflow Acoustic Sensor – Product Description 197
University of Minnesota Pipeline Products & Ongoing Clinical Trials Overview 198
Enhanced Airway Device – Product Status 198
Enhanced Airway Device – Product Description 198
Intubation Tube – Product Status 199
Intubation Tube – Product Description 199
University of Missouri Pipeline Products & Ongoing Clinical Trials Overview 200
Surgical Mouth Gag – Product Status 200
Surgical Mouth Gag – Product Description 200
University of Nebraska Pipeline Products & Ongoing Clinical Trials Overview 201
Intubating Tongue Retractor – Product Status 201
Intubating Tongue Retractor – Product Description 201
Ventilating Push Rod – Product Status 202
Ventilating Push Rod – Product Description 202
University of Pittsburgh Pipeline Products & Ongoing Clinical Trials Overview 203
Biodegradable Magnesium-Alloy Tracheal Stent – Product Status 203
Biodegradable Magnesium-Alloy Tracheal Stent – Product Description 203
University of Regensburg Pipeline Products & Ongoing Clinical Trials Overview 204
Tongue Implant – Product Status 204
Tongue Implant – Product Description 204
University of Saskatchewan Pipeline Products & Ongoing Clinical Trials Overview 205
MAIRWAY – Product Status 205
MAIRWAY – Product Description 205
University of South Florida Pipeline Products & Ongoing Clinical Trials Overview 206
Sensor Based Anesthesiology Measurement and Control System – Product Status 206
Sensor Based Anesthesiology Measurement and Control System – Product Description 206
University of Texas at San Antonio Pipeline Products & Ongoing Clinical Trials Overview 207
Breathing Tube – Product Status 207
Breathing Tube – Product Description 207
University of Texas Health Science Center at Houston Pipeline Products & Ongoing Clinical Trials Overview 208
Cuff-Less Pharyngeal Airway Management Device – Product Status 208
Cuff-Less Pharyngeal Airway Management Device – Product Description 208
Dual Function Airway Guide – Product Status 209
Dual Function Airway Guide – Product Description 209
University of Virginia Pipeline Products & Ongoing Clinical Trials Overview 210
Double-Lumen Endotracheal Tube – Product Status 210
Double-Lumen Endotracheal Tube – Product Description 210
VA Long Beach Healthcare System Pipeline Products & Ongoing Clinical Trials Overview 211
Injectable Microstimulator System – Product Status 211
Injectable Microstimulator System – Product Description 211
Vanderbilt University Pipeline Products & Ongoing Clinical Trials Overview 212
Articulating Laryngeal Mask Airway – Product Status 212
Articulating Laryngeal Mask Airway – Product Description 212
Tracheostomy Tube – Product Status 213
Tracheostomy Tube – Product Description 213
Vanderbilt University Medical Center Pipeline Products & Ongoing Clinical Trials Overview 214
Bougie – Product Status 214
Bougie – Product Description 214
Vida Medical Devices Inc Pipeline Products & Ongoing Clinical Trials Overview 215
ARDS Tube – Product Status 215
ARDS Tube – Product Description 215
Hemoblocker – Product Status 216
Hemoblocker – Product Description 216
Vincent Medical Holdings Ltd Pipeline Products & Ongoing Clinical Trials Overview 217
LiqFree – Product Status 217
LiqFree – Product Description 217
Virginia Commonwealth University Pipeline Products & Ongoing Clinical Trials Overview 218
Pressure Sensing Endotracheal Tube – Product Status 218
Pressure Sensing Endotracheal Tube – Product Description 218
Vrije University Brussel Pipeline Products & Ongoing Clinical Trials Overview 219
Endotracheal Tube – Product Status 219
Endotracheal Tube – Product Description 219
Wake Forest Baptist Medical Center Pipeline Products & Ongoing Clinical Trials Overview 220
Endotracheal Tube Exchanger – Product Status 220
Endotracheal Tube Exchanger – Product Description 220
Locking Ventilation Bag – Product Status 221
Locking Ventilation Bag – Product Description 221
Walter Cantidio University Hospital Pipeline Products & Ongoing Clinical Trials Overview 222
COVID-Box – Product Status 222
COVID-Box – Product Description 222
WynnVision LLC Pipeline Products & Ongoing Clinical Trials Overview 223
Endotracheal Tube – Product Status 223
Endotracheal Tube – Product Description 223
Xhale Inc Pipeline Products & Ongoing Clinical Trials Overview 224
Assurance Pharmacodynamic Anesthesia Administration Monitor – Product Status 224
Assurance Pharmacodynamic Anesthesia Administration Monitor – Product Description 224
Youyi Medical Instrument Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 225
SaCoVLM Video Laryngeal Mask Airway – Product Status 225
SaCoVLM Video Laryngeal Mask Airway – Product Description 225
Youyi Medical Instrument Co Ltd – Ongoing Clinical Trials Overview 226
SaCoVLM Video Laryngeal Mask Airway – Clinical Effect Analysis of SaCoVLM Video Laryngeal Mask Guided Intubation and UE Video Laryngoscope Intubation in General Anesthesia Airway Management 227
SaCoVLM Video Laryngeal Mask Airway – Evaluation of SaCo Videolaryngeal Mask Airway for Airway Management and Intubation in Morbidly Obese Anesthetized for Elective Surgery. 227
SaCoVLM Video Laryngeal Mask Airway – Intubation Using Video Laryngeal Mask Airway SaCoVLM and Laryngeal Mask Airway Ambu Aura-i in Anesthetized Children 227
SaCoVLM Video Laryngeal Mask Airway – The Stylet Facilitates the Insertion of SaCo Laryngeal Mask 228
Zennea Technologies Pipeline Products & Ongoing Clinical Trials Overview 229
ZENS – Product Status 229
ZENS – Product Description 229
Glossary 282
![]()