Artificial Pancreas Pipeline by Development Stages, Segments, Region and Countries, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Artificial Pancreas Pipeline Market Report Overview
The artificial pancreas aids people in controlling their blood glucose level or diabetes, primarily type 1, automatically by continuously providing the substitute endocrine functionality of a healthy pancreas.
The artificial pancreas pipeline market research report provides comprehensive information about the products with their comparative segment-wise analysis at various stages of development. The report also covers the regional and country-wise artificial pancreas clinical trials outlook. The regulatory paths followed for conducting the artificial pancreas pipeline research are extensively highlighted in the report. Leading companies involved in the artificial pancreas pipeline development are also enlisted in this research report for better understanding of the competitive landscape.
| Key Territories | · The US
· Europe · China · Australia · Canada · Japan · South Korea |
| Key Regulatory Paths | · PMA
· CE · NMPA · 510(k) · Shonin · TGA |
| Leading Companies | · Admetsys Corp
· Beta Bionics Inc · Bigfoot Biomedical Inc · BioTex Inc · Cerco Medical LLC |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Artificial Pancreas Pipeline Market Segmentation by Territories
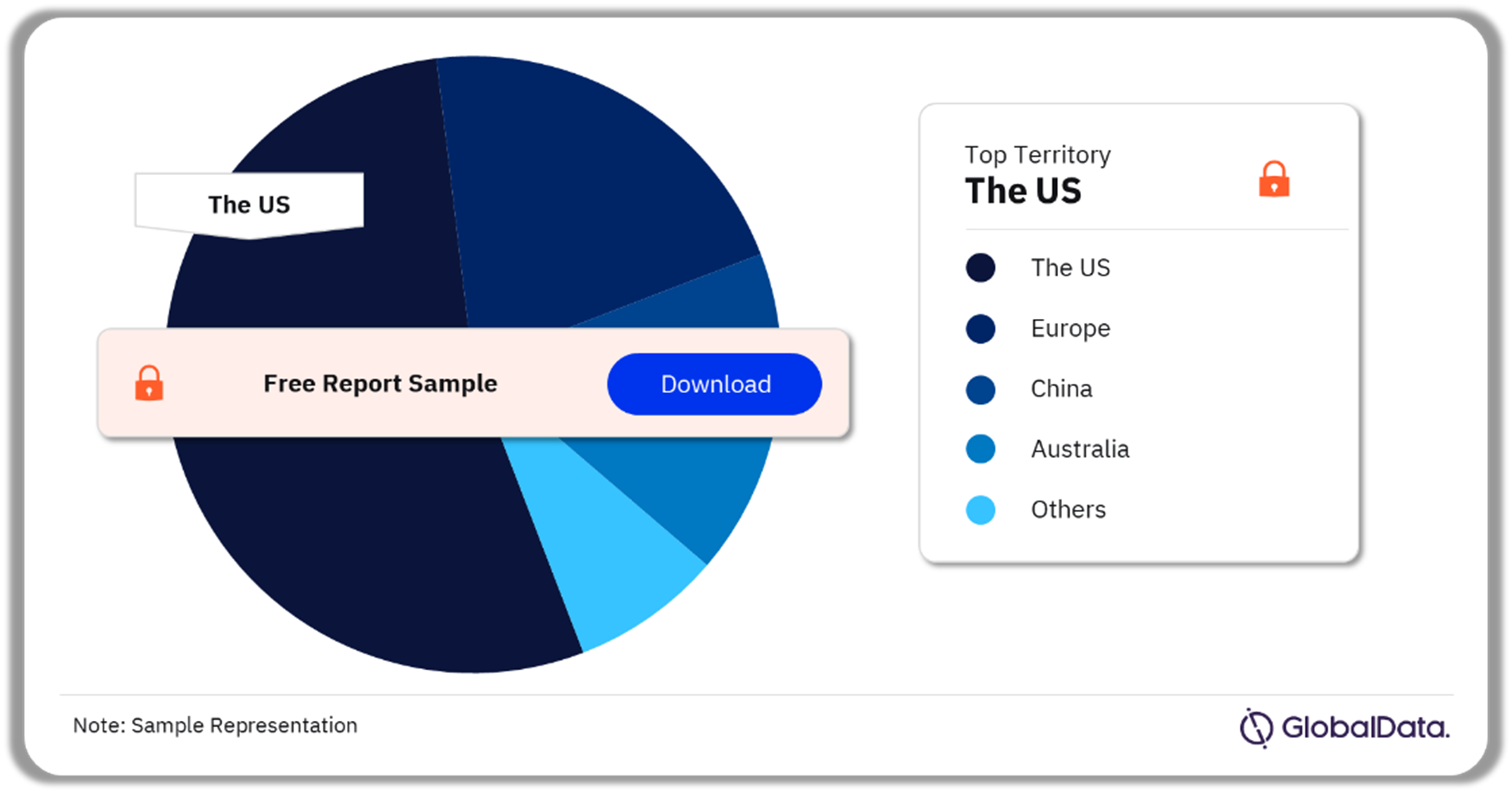
The key territories in the artificial pancreas market are the US, Europe, China, Australia, Canada, Japan, and South Korea. As of February 2024, the US accounted for the highest number of pipeline products.
Artificial Pancreas Pipeline Market Analysis by Territories, 2023 (%)
Buy the Full Report for More Territory Insights into the Artificial Pancreas Pipeline Market
Artificial Pancreas Pipeline Market Segmentation by Regulatory Paths
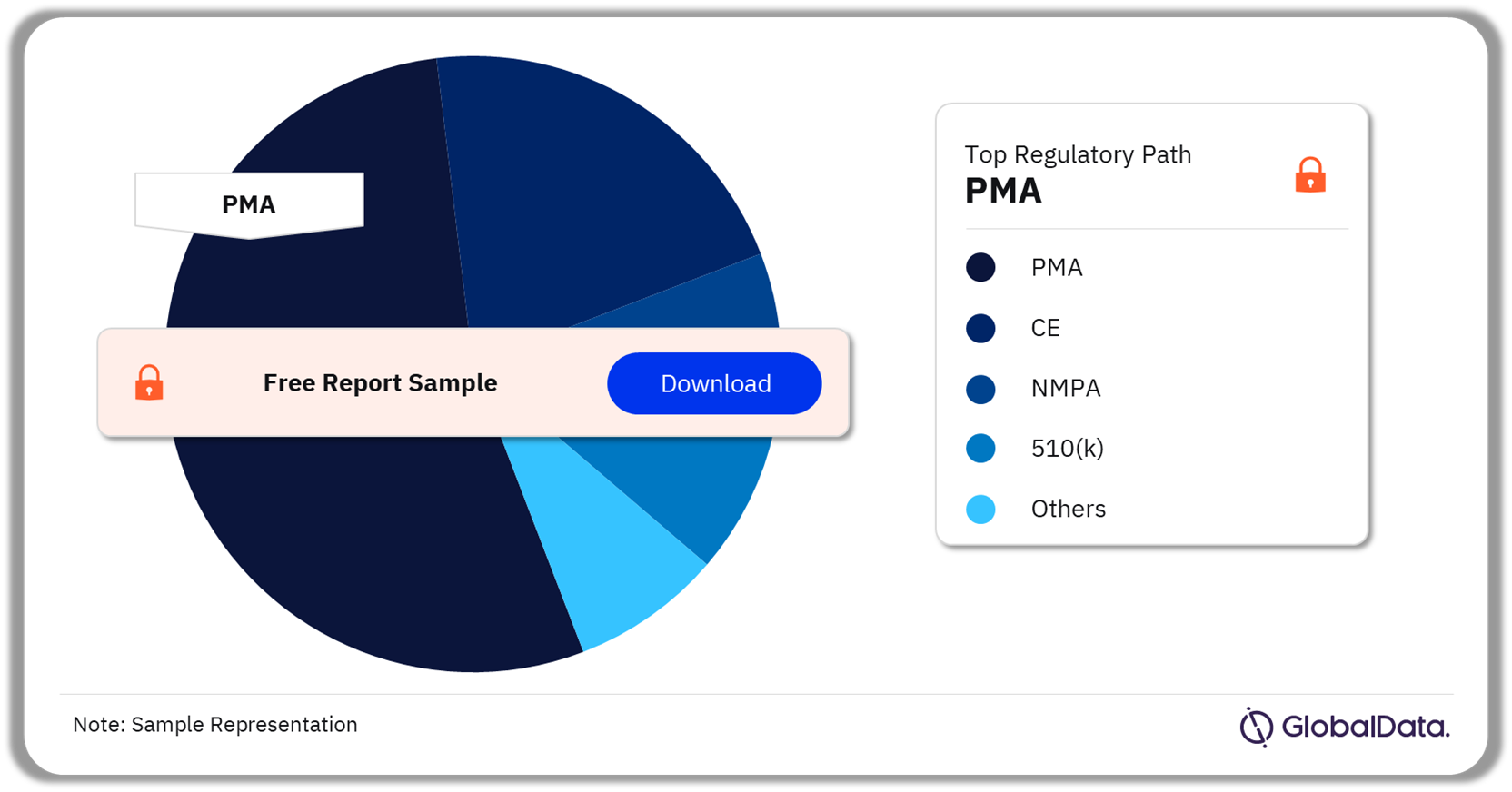
The key regulatory paths in the artificial pancreas pipeline market are PMA, CE, NMPA, 510(k), Shonin, and TGA among others. As of February 2024, PMA was the most followed pathway for pipeline products in the market.
Artificial Pancreas Pipeline Market Analysis by Regulatory Paths, 2023 (%)
Buy the Full Report for More Regulatory Path Insights into the Artificial Pancreas Pipeline Market
Artificial Pancreas Pipeline Market – Competitive Landscape
The leading companies in the artificial pancreas pipeline market are Admetsys Corp, Advanced Biosensors-Ohio LLC, Beta Bionics Inc, and Bigfoot Biomedical Inc among others.
Admetsys Corp (Admetsys): Admetsys Corp (Admetsys) provides artificial pancreas devices for surgical care and hospitals to counterbalance the treatment of glucose and insulin. It offers its solutions for physicians, nurses, and administrators. The company’s key product in this category is the Smart Pancreas, a real-time, fully automated blood glucose control system intended for diabetes management. It also maintains constant blood sugar levels.
Leading Artificial Pancreas Pipeline Market Companies, 2023
Buy the Full Report for More Company Insights into the Artificial Pancreas Pipeline Market
Segments Covered in the Report
Artificial Pancreas Pipeline Market Territories Outlook
- The US
- Europe
- China
- Australia
- Canada
- Japan
- South Korea
Artificial Pancreas Pipeline Market Regulatory Paths Outlook
- PMA
- CE
- NMPA
- 510(k)
- Shonin
- TGA
Scope
This report provides:
- Extensive coverage of the artificial pancreas under development.
- Details of major pipeline products that include product description, licensing and collaboration details and other developmental activities.
- Reviews of major players involved in the development of artificial pancreas and lists all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from early development to approved/issued stage.
- Provides key clinical trial data of ongoing clinical trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with potentially strong product portfolios and create effective counter strategies to gain a competitive advantage.
- Identify and understand important and diverse types of Artificial pancreas under development.
- Develop market-entry and market-expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date.
Advanced Biosensors-Ohio LLC
AiTA Bio Inc
Beta Bionics Inc
Beta-O2 Technologies Ltd
Bigfoot Biomedical Inc
BioTex Inc
Captix Biomedical Pty Ltd
Cerco Medical LLC
Children's Hospital of Philadelphia
Converge Biotech, Inc.
Covalor Medical, LLC
De Montfort University
Debiotech SA
Defymed SAS
Diabeloop SA
EOFlow Co Ltd
Giner Inc
Harvard John A Paulson School of Engineering and Applied Sciences
Healios Inc
Humacyte Inc
Ideal Medical Technologies Inc
Imperial College London
Indian Institute of Science
Inreda Diabetic BV
Joslin Diabetes Center
Kailian Medical Technology Co Ltd
Kencak LLC
McGill University
Medtronic Inc
Medtronic MiniMed Inc
MicroTech Medical Co Ltd
Miromatrix Medical Inc
Modular Medical Inc
Pacific Diabetes Technologies Inc
Pancreum, LLC
PharmaCyte Biotech Inc
Polytechnic University of Valencia
Rensselaer Polytechnic Institute
Senseonics Holdings Inc
Shenzhen Huayuan Regenerative Medicine Co Ltd
Stanford University
TecMed Inc
TypeZero Technologies LLC
U-Needle BV
Universidad Autonoma de Madrid
University of California San Francisco
University of California Santa Barbara
University of Cambridge
University of Florida
University of Minnesota
University of Newcastle
University of Pennsylvania
University of Queensland
WaveForm Technologies Inc
Xeris Biopharma Holdings Inc
Yale University
Ypsomed Holding AG
Table of Contents
Table
Figures
Frequently asked questions
-
Which territory had the highest number of products in the artificial pancreas pipeline market as of February 2024?
As of February 2024, the US had the highest number of products in the artificial pancreas pipeline market.
-
Which was the most followed regulatory pathway in the artificial pancreas pipeline market as of February 2024?
As of February 2024, PMA was the most followed pathway for pipeline products in the artificial pancreas market.
-
Which are the major companies operating in the artificial pancreas pipeline market?
The key companies in the artificial pancreas pipeline market are Admetsys Corp, Advanced Biosensors-Ohio LLC, Beta Bionics Inc., and Bigfoot Biomedical Inc. among others.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Artificial pancreas reports