Bile Duct Cancer (Cholangiocarcinoma) Drugs in Development by Stages, Target, MoA, RoA, Molecule Type and Key Players, 2022 Update
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Bile Duct Cancer Pipeline Drugs Market Report Overview
Bile duct cancer (cholangiocarcinoma) are tumors that occur in the bile duct. Symptoms include discomfort in the tummy area (abdomen), loss of appetite, high temperatures (fevers), and weight loss. Treatment includes chemotherapy and radiation therapy.
The Bile Duct Cancer drugs in development market research report provides comprehensive information on the therapeutics under development for Bile Duct Cancer, complete with analysis by stage of development, drug target, mechanism of action (MoA), route of administration (RoA), and molecule type. The report also covers the descriptive pharmacological action of the therapeutics, its complete research and development history, and the latest news and press releases. Additionally, the report provides an overview of key players involved in therapeutic development for Bile Duct Cancer and features dormant and discontinued projects.
Bile Duct Cancer Pipeline Drugs Market Segmentation by Targets
Some of the targets in the Bile Duct Cancer pipeline are Programmed Cell Death 1 Ligand 1, Fibroblast Growth Factor Receptor 2, Fibroblast Growth Factor Receptor 3, and Fibroblast Growth Factor Receptor 1 among others. Programmed Cell Death 1 Ligand 1 was the largest target in the pipeline.
Bile Duct Cancer Pipeline Drugs Market Analysis, by Targets
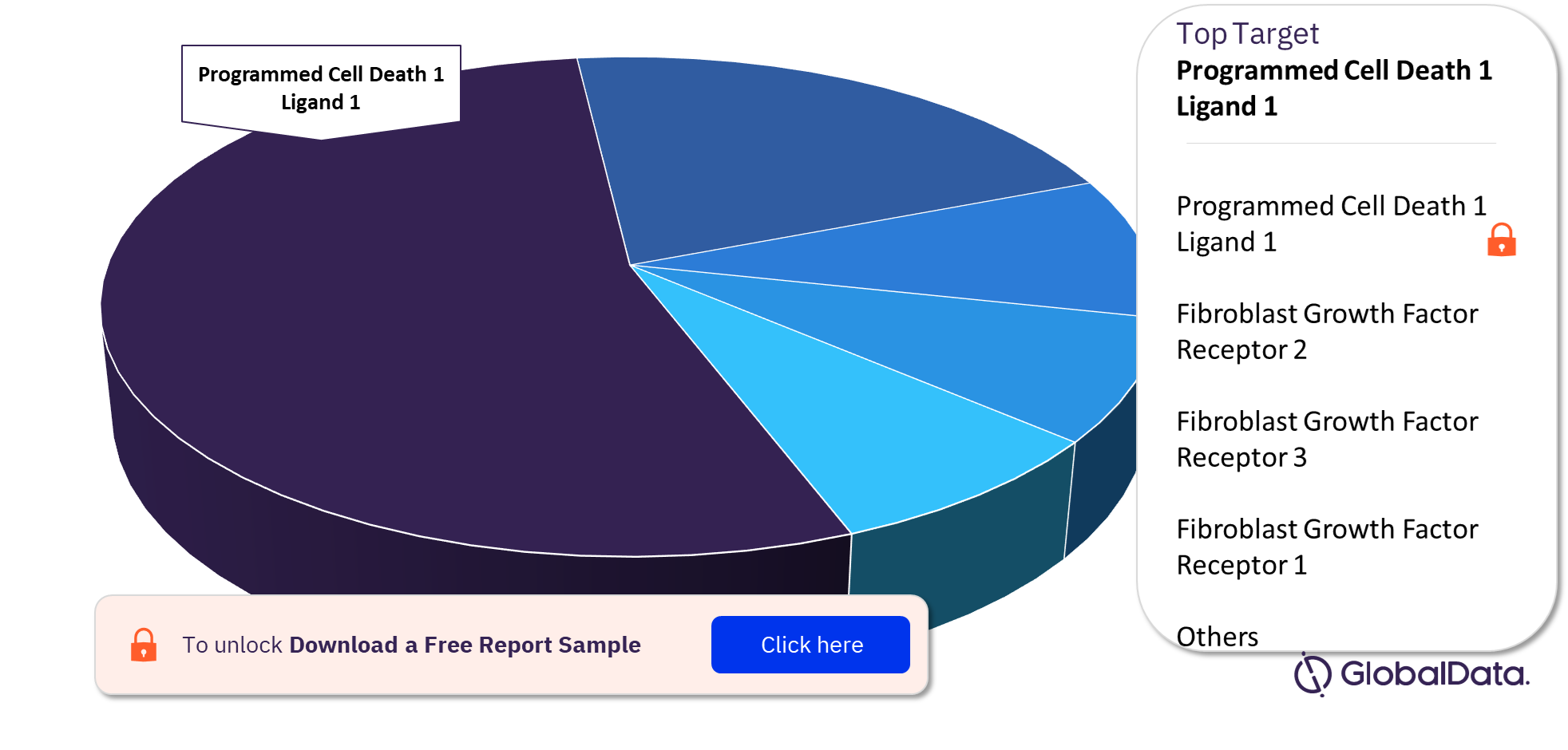 For more Bile Duct Cancer pipeline drugs market target insights, download a free report sample
For more Bile Duct Cancer pipeline drugs market target insights, download a free report sample
Bile Duct Cancer Pipeline Drugs Market Segmentation by Mechanisms of Action
Some of the mechanisms of action in the Bile Duct Cancer pipeline drugs market are Programmed Cell Death 1 Ligand 1 Inhibitor, Fibroblast Growth Factor Receptor 2 Inhibitor, Fibroblast Growth Factor Receptor 3 Inhibitor, and Fibroblast Growth Factor Receptor 1 Inhibitor among others. Programmed Cell Death 1 Ligand 1 Inhibitor was the leading MoA in the pipeline.
Bile Duct Cancer Pipeline Drugs Market Analysis, by Mechanisms of Action
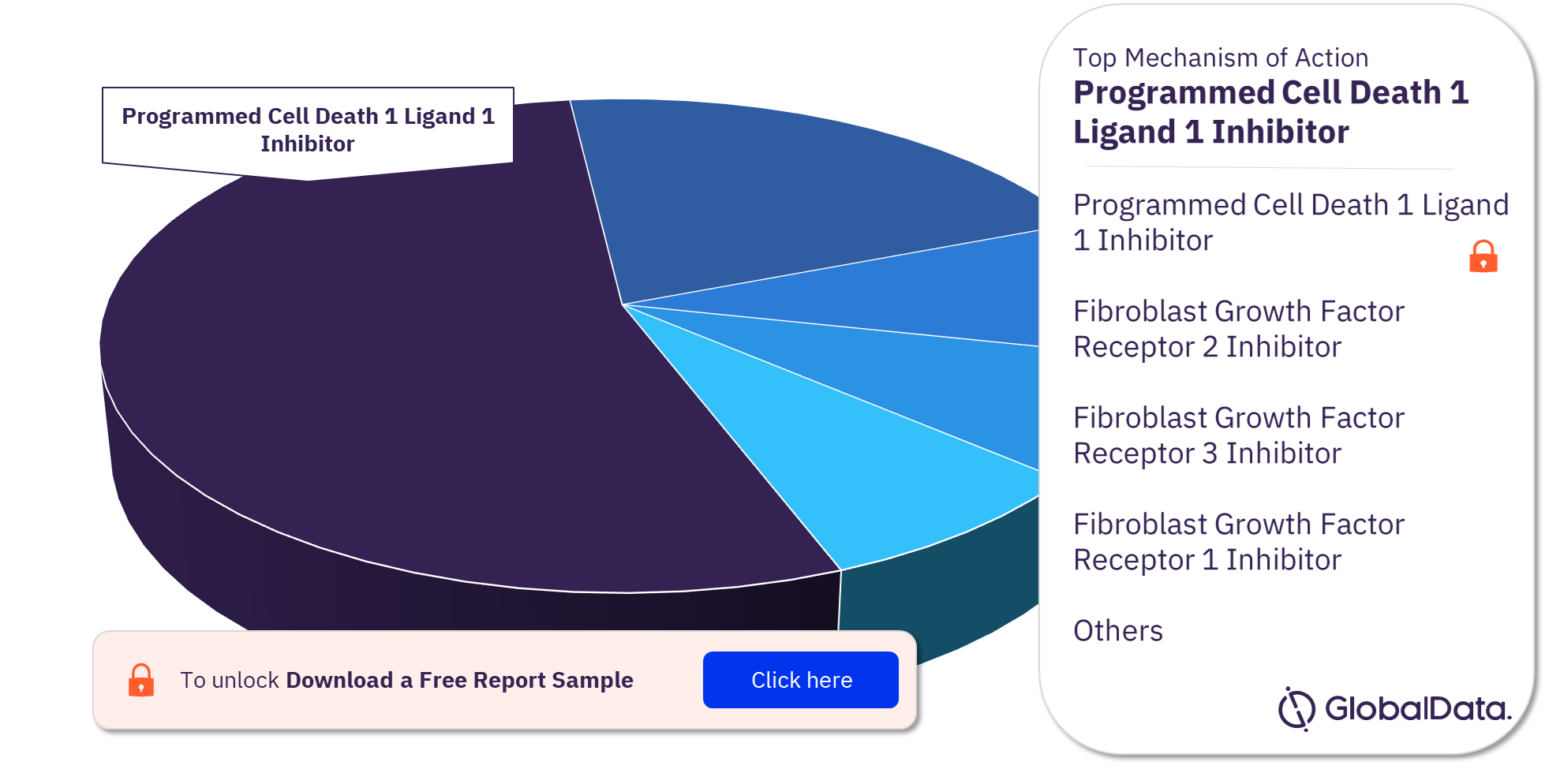 For more MoA insights into the Bile Duct Cancer pipeline drugs market, download a free report sample
For more MoA insights into the Bile Duct Cancer pipeline drugs market, download a free report sample
Bile Duct Cancer Pipeline Drugs Market Segmentation by Routes of Administration
The key routes of administration in the Bile Duct Cancer pipeline drugs market are intravenous, oral, subcutaneous, and intravenous drip among others. The majority of the pipeline drugs followed the intravenous route of administration.
Bile Duct Cancer Pipeline Drugs Market Analysis, by Routes of Administration
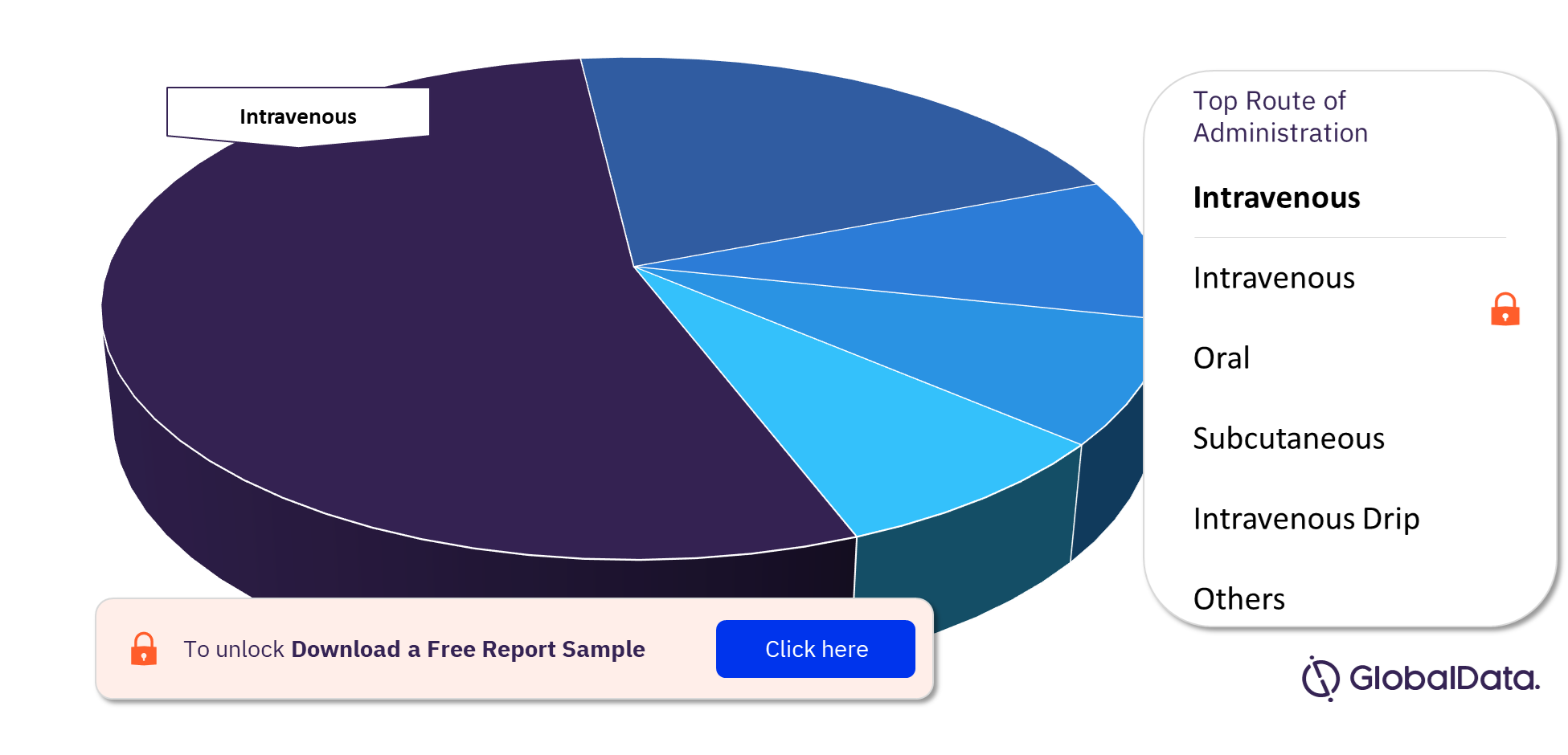 For more routes of administration insights into the Bile Duct Cancer pipeline drugs market, download a free report sample
For more routes of administration insights into the Bile Duct Cancer pipeline drugs market, download a free report sample
Bile Duct Cancer Pipeline Drugs Market Segmentation by Molecule Types
The leading molecule types in the Bile Duct Cancer pipeline drugs market are small molecule, monoclonal antibody, gene-modified cell therapy, and monoclonal antibody conjugated among others. Small molecule was the leading molecule type in the pipeline.
Bile Duct Cancer Pipeline Drugs Market Analysis, by Molecule Types
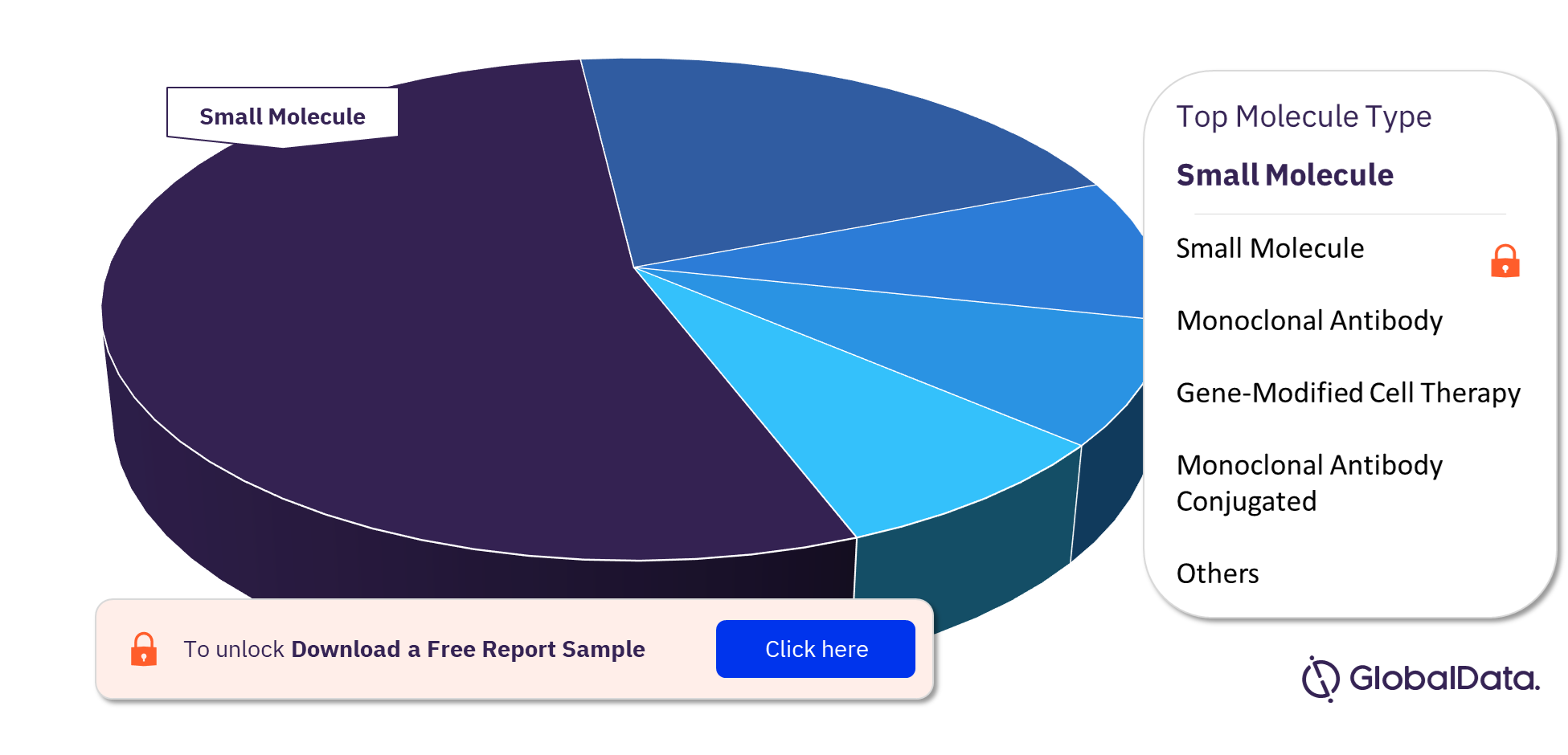 For more molecule type insights into the Bile Duct Cancer pipeline drugs market, download a free report sample
For more molecule type insights into the Bile Duct Cancer pipeline drugs market, download a free report sample
Bile Duct Cancer Pipeline Drugs Market - Competitive Landscape
Some of the key companies in the Bile Duct Cancer pipeline drugs market are AstraZeneca Plc, Bayer AG, Merck & Co Inc, and Advenchen Laboratories LLC among others. Among these, AstraZeneca Plc had the most number of products under development in 2022.
Bile Duct Cancer Pipeline Drugs Market Analysis by Key Companies
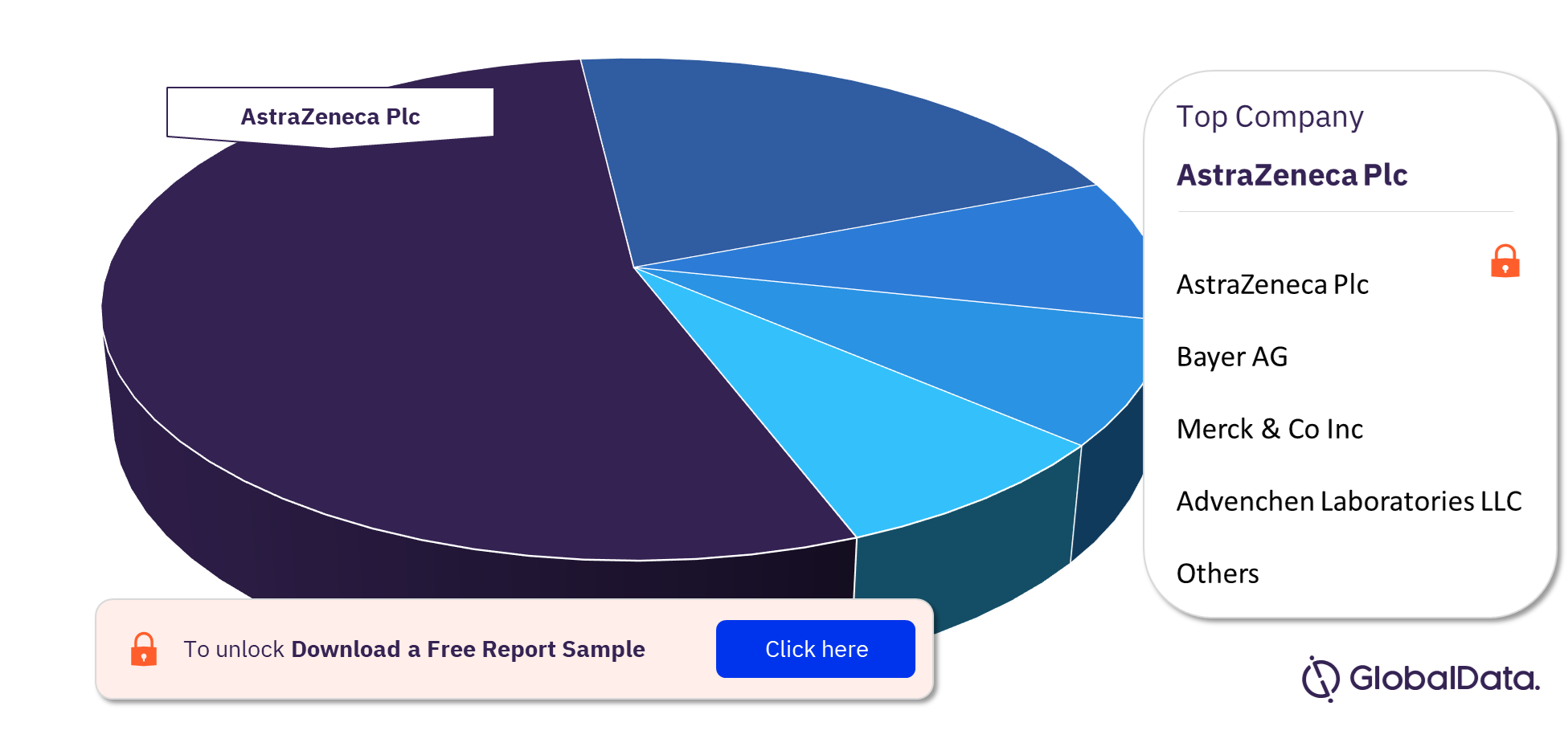 To know more about the Bile Duct Cancer pipeline drugs market companies, download a free report sample
To know more about the Bile Duct Cancer pipeline drugs market companies, download a free report sample
Bile Duct Cancer Pipeline Drugs Market Report Overview
| Key Targets | Programmed Cell Death 1 Ligand 1, Fibroblast Growth Factor Receptor 2, Fibroblast Growth Factor Receptor 3, and Fibroblast Growth Factor Receptor 1 |
| Key Mechanism of Actions | Programmed Cell Death 1 Ligand 1 Inhibitor, Fibroblast Growth Factor Receptor 2 Inhibitor, Fibroblast Growth Factor Receptor 3 Inhibitor, and Fibroblast Growth Factor Receptor 1 Inhibitor |
| Key Routes of Administration | Intravenous, Oral, Subcutaneous, and Intravenous Drip |
| Key Molecule Types | Small Molecule, Monoclonal Antibody, Gene-Modified Cell Therapy, and Monoclonal Antibody Conjugated |
| Key Companies | AstraZeneca Plc, Bayer AG, Merck & Co Inc, and Advenchen Laboratories LLC |
Scope
- The pipeline guide provides a snapshot of the global therapeutic landscape of Bile Duct Cancer (Cholangiocarcinoma).
- The pipeline guide reviews pipeline therapeutics for Bile Duct Cancer (Cholangiocarcinoma) by companies and universities/research institutes based on information derived from company and industry-specific sources.
- The pipeline guide covers pipeline products based on several stages of development ranging from pre-registration till discovery and undisclosed stages.
- The pipeline guide features descriptive drug profiles for the pipeline products which comprise, product description, descriptive licensing and collaboration details, R&D brief, MoA & other developmental activities.
- The pipeline guide reviews key companies involved in Bile Duct Cancer (Cholangiocarcinoma) therapeutics and enlists all their major and minor projects.
- The pipeline guide evaluates Bile Duct Cancer (Cholangiocarcinoma) therapeutics based on mechanism of action (MoA), drug target, route of administration (RoA) and molecule type.
- The pipeline guide encapsulates all the dormant and discontinued pipeline projects.
- The pipeline guide reviews latest news related to pipeline therapeutics for Bile Duct Cancer (Cholangiocarcinoma)
Reasons to Buy
- Procure strategically important competitor information, analysis, and insights to formulate effective R&D strategies.
- Recognize emerging players with potentially strong product portfolio and create effective counter-strategies to gain competitive advantage.
- Find and recognize significant and varied types of therapeutics under development for Bile Duct Cancer (Cholangiocarcinoma).
- Classify potential new clients or partners in the target demographic.
- Develop tactical initiatives by understanding the focus areas of leading companies.
- Plan mergers and acquisitions meritoriously by identifying key players and it’s most promising pipeline therapeutics.
- Formulate corrective measures for pipeline projects by understanding Bile Duct Cancer (Cholangiocarcinoma) pipeline depth and focus of Indication therapeutics.
- Develop and design in-licensing and out-licensing strategies by identifying prospective partners with the most attractive projects to enhance and expand business potential and scope.
- Adjust the therapeutic portfolio by recognizing discontinued projects and understand from the know-how what drove them from pipeline. Plan mergers and acquisitions meritoriously by identifying key players and it’s most promising pipeline therapeutics.
- Formulate corrective measures for pipeline projects by understanding Bile Duct Cancer (Oncology) pipeline depth and focus of Indication therapeutics.
- Develop and design in-licensing and out-licensing strategies by identifying prospective partners with the most attractive projects to enhance and expand business potential and scope.
- Adjust the therapeutic portfolio by recognizing discontinued projects and understand from the know-how what drove them from pipeline.
AbbVie Inc
Ability Pharmaceuticals SL
ABL Bio Inc
ABM Therapeutics Inc
ADC Therapeutics SA
Advenchen Laboratories LLC
Affimed GmbH
Akeso Inc
Alaunos Therapeutics Inc
Alentis Therapeutics AG
Alkermes Plc
Alligator Bioscience AB
Alphamab Oncology
Alpine Immune Sciences Inc
Ambrx Biopharma Inc
Amgen Inc
AngioGenex Inc
Apexigen Inc
Apollomics Inc
Arbele Ltd
Ascelia Pharma AB
Aslan Pharmaceuticals Ltd
AstraZeneca Plc
Bavarian Nordic AS
Bayer AG
BeiGene Ltd
Beijing Biostar Technologies Ltd
Beijing Immunochina Pharmaceuticals Co Ltd
Betta Pharmaceuticals Co Ltd
BioMed Valley Discoveries Inc
BioNTech SE
Biosion Inc
Biotheus Inc
BJ Bioscience Inc
Blueprint Medicines Corp
Boehringer Ingelheim International GmbH
Bold Therapeutics Inc
Bristol-Myers Squibb Co
CanBas Co Ltd
Celldex Therapeutics Inc
Celltrion Inc
Celon Pharma SA
Chong Kun Dang Pharmaceutical Corporation
Corcept Therapeutics Inc
Cornerstone Pharmaceuticals Inc
CSPC Pharmaceutical Group Ltd
CStone Pharmaceuticals Co Ltd
Cyclacel Pharmaceuticals Inc
CytoImmune Therapeutics Inc
Daiichi Sankyo Co Ltd
Delcath Systems Inc
Delta-Fly Pharma Inc
Duo Oncology Inc
Eddingpharm Inc
Eisai Co Ltd
Elevation Oncology Inc
Eli Lilly and Co
Elucida Oncology Inc
EpimAb Biotherapeutics Inc
Exelixis Inc
F. Hoffmann-La Roche Ltd
Faron Pharmaceuticals Oy
Fujifilm Holdings Corp
Genenta Science SpA
Genentech USA Inc
GeneQuantum Healthcare Suzhou Co Ltd
Genexine Inc
GenFleet Therapeutics (Shanghai) Inc
Genome & Co
Genoscience Pharma
Genosco Inc
Gensun Biopharma Inc
GI Innovation Co Ltd
GO Therapeutics Inc
GSK plc
Guangdong Zhongsheng Pharmaceutical Co Ltd
H3 Biomedicine Inc
Hangzhou Bensheng Pharmaceutical Co Ltd
Harbin Gloria Pharmaceuticals Co Ltd
Harbour BioMed (Guangzhou) Co Ltd
Heidelberg Pharma AG
Heilongjiang ZBD Pharmaceutical Co Ltd
Hutchison MediPharma Ltd
Ikena Oncology Inc
ImCare Biotech LLC
Immix BioPharma Inc
ImmuneOnco Biopharmaceuticals (Shanghai) Co Ltd
Immunitor Inc
Incyte Corp
Inmune Bio Inc
InnoCare Pharma Ltd
Innopharmax Inc
Innovative Cellular Therapeutics Co Ltd
Innovent Biologics Inc
Intensity Therapeutics Inc
Ipsen SA
Jiangsu Hengrui Medicine Co Ltd
Johnson & Johnson
Kinnate Biopharma Inc
Komipharm International Co Ltd
KPC Pharmaceuticals Inc
LaNova Medicines Ltd
Leap Therapeutics Inc
LegoChem Biosciences Inc
Les Laboratoires Servier SAS
Ligand Pharmaceuticals Inc
LipoMedix Pharmaceutical Inc
Luzitin SA
MacroGenics Inc
MedAnnex Ltd
Medicenna Therapeutics Corp
MediciNova Inc
Medivir AB
Merck & Co Inc
Merck KGaA
Merus NV
Mina Therapeutics Ltd
Mirati Therapeutics Inc
Molecular Templates Inc
Nanjing KAEDI Biotech Inc
NanoCarrier Co Ltd
Nerviano Medical Sciences SRL
Netherlands Translational Research Center BV
NGM Biopharmaceuticals Inc
Nkarta Inc
Northwest Biotherapeutics Inc
Novartis AG
NuCana Plc
Nuvalent Inc
Orgenesis Inc
Otsuka Pharmaceutical Co Ltd
Perseus Proteomics Inc
Pfizer Inc
Pharma Mar SA
Pharmaxis Ltd
Pieris Pharmaceuticals Inc
Promontory Therapeutics Inc
Provecs Medical GmbH
Puma Biotechnology Inc
Puretech Health Plc
QED Therapeutics Inc
Quadriga BioSciences Inc
QureBio Ltd
Recordati SpA
RedHill Biopharma Ltd
Redx Pharma Plc
Relay Therapeutics Inc
RemeGen Co Ltd
Rhizen Pharmaceuticals SA
Rigel Pharmaceuticals Inc
Samyang Biopharmaceuticals Corp
Sanofi
Seagen Inc
Senhwa Biosciences Inc
Shandong Boan Biotechnology Co Ltd
Shandong Buchang Pharmaceutical Co Ltd
Shanghai Cell Therapy Group Co
Shanghai Fosun Pharmaceutical (Group) Co Ltd
Shanghai Haihe Biopharma Co Ltd
Shanghai Junshi Bioscience Co Ltd
Shanghai Miracogen Inc
Shanghai Novamab Biopharmaceuticals Co Ltd
Shanghai Yingli Pharmaceutical Co Ltd
Shouyao Holding Co Ltd
Sihuan Pharmaceutical Holdings Group Ltd
Sillajen Biotherapeutics
Sirnaomics Ltd
Sorrento Therapeutics Inc
Starpharma Holdings Ltd
SupremeCure Pharma Inc
Suzhou Kebo Ruijun Biosciences Co Ltd
Suzhou Zelgen Biopharmaceutical Co Ltd
Symphogen A/S
SynCore Biotechnology Co Ltd
Syndax Pharmaceuticals Inc
SyntheX Inc
Taiho Oncology Inc
Tango Therapeutics Inc
TCR2 Therapeutics Inc
Tempest Therapeutics Inc
Toray Industries Inc
Transcenta Holding Ltd
TransThera Sciences (Nanjing) Inc
TriSalus Life Sciences Inc
Tyra Biosciences Inc
VasGene Therapeutics Inc
Verismo Therapeutics
Virogin Biotech Ltd
Vivesto AB
WellMarker Bio Co Ltd
Xencor Inc
Zhangzhou Pien Tze Huang Pharmaceutical Co Ltd
Zhejiang Borui Biopharmaceutical Co Ltd
Zymeworks Inc
Table of Contents
Table
Figures
Frequently asked questions
-
What are the key targets of the Bile Duct Cancer pipeline drugs market?
Some of the key targets of the Bile Duct Cancer pipeline drugs market are Programmed Cell Death 1 Ligand 1, Fibroblast Growth Factor Receptor 2, Fibroblast Growth Factor Receptor 3, and Fibroblast Growth Factor Receptor 1.
-
What are the key mechanisms of action in the Bile Duct Cancer pipeline drugs market?
Some of the key mechanisms of action in the Bile Duct Cancer pipeline drugs market are Programmed Cell Death 1 Ligand 1 Inhibitor, Fibroblast Growth Factor Receptor 2 Inhibitor, Fibroblast Growth Factor Receptor 3 Inhibitor, and Fibroblast Growth Factor Receptor 1 Inhibitor.
-
What are the key routes of administration in the Bile Duct Cancer pipeline drugs market?
The key routes of administration in the Bile Duct Cancer pipeline drugs market are intravenous, oral, subcutaneous, and intravenous drip.
-
What are the key molecule types in the Bile Duct Cancer pipeline drugs market?
The molecule types in the Bile Duct Cancer pipeline drugs market are small molecule, monoclonal antibody, gene-modified cell therapy, and monoclonal antibody conjugated among others.
-
Which are the key companies in the Bile Duct Cancer pipeline drugs market?
Some of the key companies in the Bile Duct Cancer pipeline drugs market are AstraZeneca Plc, Bayer AG, Merck & Co Inc, and Advenchen Laboratories LLC among others.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Oncology reports








