Biosimilars in Oncology – Thematic Intelligence
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Accessing in-depth insights from the ‘Biosimilars in Oncology’ report will help you:
- Understand the milestones of biosimilars in oncology, the trends in the market, and the biosimilar value chain.
- Assess the marketed and pipeline products and their uptake across 8MM.
- Learn about the percentage of cancer patients treated with biosimilars, the price of the agents compared to their originator, and key opinion leaders’ outlook for the products.
- Examine the 8MM regulatory landscapes, their market potential, and their available and upcoming biosimilars.
- Analyze the opportunities, challenges, and unmet needs facing biosimilar uptake and commercially assessing the major current and future players in the field.
How is our ‘Biosimilars in Oncology’ report unique from other reports in the market?
- The report provides biosimilars in oncology market assessment across multiple regions.
- The report provides an insight into approaches required to promote biosimilar uptake.
- Our thematic report answer questions such as:
-
- What is the percentage of cancer patients treated with biosimilars in each 8MM country?
- Which patient groups are more likely to receive these therapies?
- What is the price of these agents compared to their originator molecule?
- What is the key opinion leaders’ outlook for the products?
- How do biosimilar discounts work and how might they affect future uptake?
We recommend this valuable source of information to anyone involved in:
- Pharmaceutical Industries (Big Pharma, Small Biotech, Start-ups, etc.),
- Pharmaceutical Industry Suppliers (e.g., Consulting Companies, CMOs, CDMOs, CROs, Technology Vendors)
- Consulting and Professional Services (e.g., Investment Companies, Investment Banks, Equity Companies, etc.)
- Pharma Manufacturers (Innovative, Biotech, Generics, Biosimilars, Rare/Orphan Disease) / Distributors
- Financial Institutions (Investors in Pharma/Biotech, Pricing Consultancies)/Parallel Trade Organisations
- Healthcare Organisations (Payers, HTA Bodies, Reimbursement Groups, Government Healthcare Organizations)
- Clinical Research Organisations (CROs)
To Get a Snapshot of the State of the Biosimilars in Oncology Report
Biosimilars in Oncology Thematic Report Overview
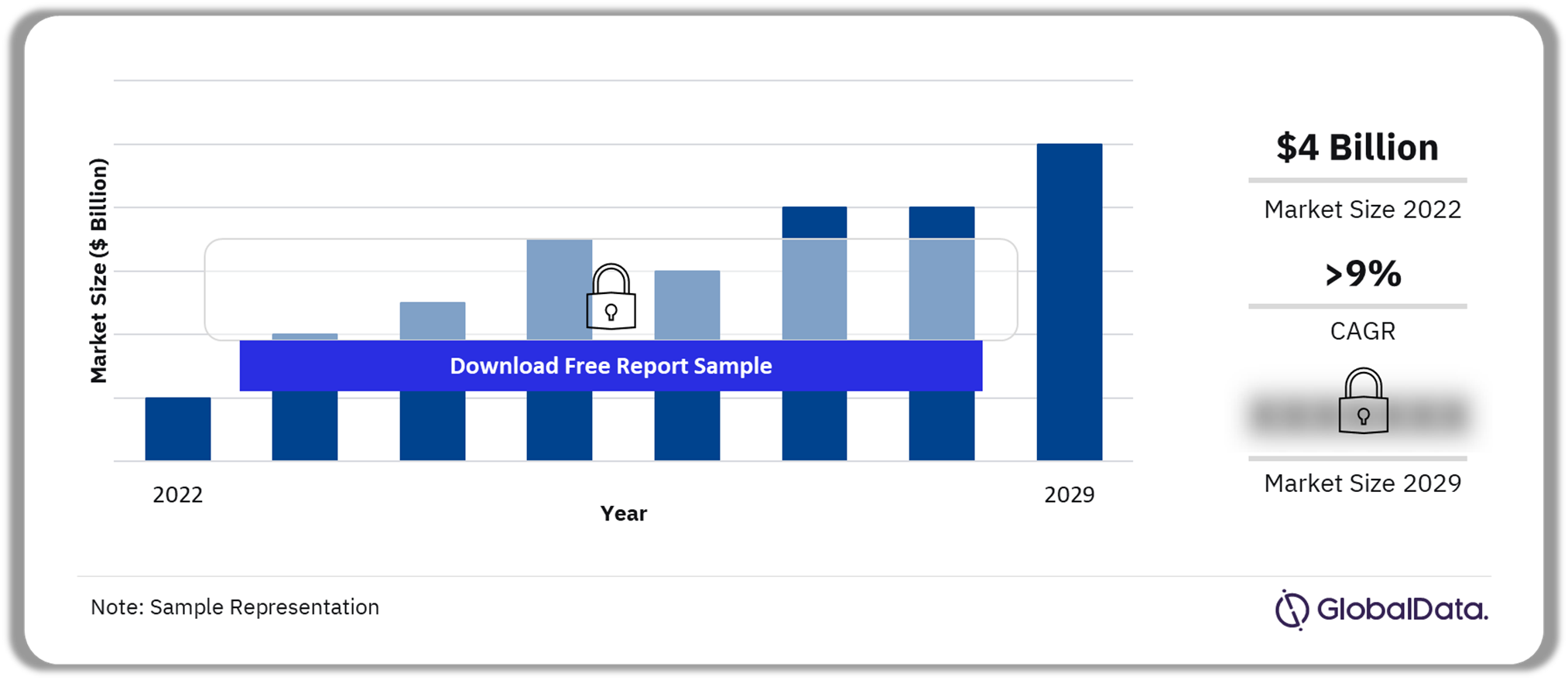
The biosimilar market size in the 8MM was $4 billion in 2022 and is expected to achieve a CAGR of more than 9% during 2022-2029. The market growth has increased sharply in the last five years due to the launch of biosimilars such as bevacizumab, rituximab, and trastuzumab in the 8MM, as well as the high adoption of these products. Biosimilars are biological drugs that are similar, but not identical, to their reference or original product. Minor variability between a biosimilar and its reference medicine is allowed. However, biosimilars should not show any clinically meaningful difference from the originator in terms of quality, safety, and efficacy.
Biosimilar Market Outlook, 2022-2029 ($ Billion)
Buy the Full Report for More Insights into the Biosimilars Market Forecast
The Biosimilars in Oncology thematic research report includes the 8MM regulatory landscapes, their market potential, and their available and upcoming biosimilars. Additionally, the report includes the opportunities and challenges facing biosimilar uptake and a commercial assessment of the major players in the field.
| Market Size (2022) | $4 billion |
| CAGR (2022-2029) | >9% |
| Historical Period | 2018-2023 |
| Forecast Period | 2024-2029 |
| Key Regions | · US
· 5EU · Japan · China |
| Key Companies | · Sandoz
· Celltrion · Amgen · Biocon · Pfizer · Organon |
| Enquire and Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before purchasing. |
Biosimilars in Oncology - Trends
Since biosimilars are complex, large molecules, they are costly to develop. Therefore, biosimilar companies offer limited discounts. However, manufacturers of the reference product offer discounts and may supply new formulations that are more convenient to use, offsetting the available discounts on biosimilars by biosimilar companies.
Additionally, competition in the biosimilars industry has decreased the cost of biosimilars and their reference products. Increasing market competition due to the availability of multiple biosimilars for a single reference product may raise pricing pressures and compel manufacturers to offer significant discounts. Such discount pricing could reduce future commercial incentives to develop biosimilars.
Buy the Full Report for More Trend Insights into the Biosimilars in Oncology Theme
Biosimilars in Oncology Market Segmentation by Regions
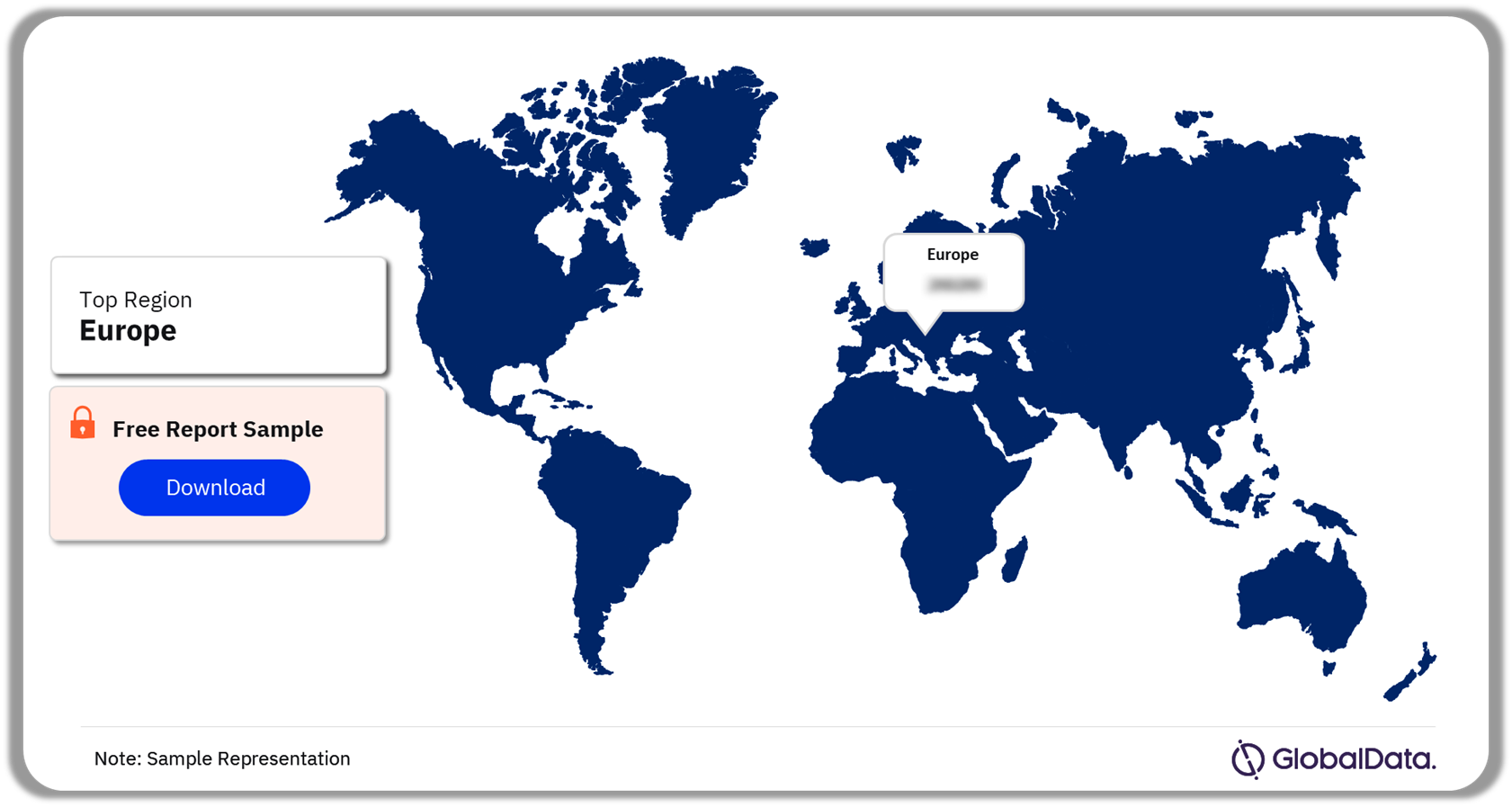
The oncology biosimilar market is prevalent in the US, 5EU, Japan, and China. Globally, Europe has the highest adoption of biosimilars as many healthcare institutions in the region only prescribe biosimilars. Moreover, in the EU, biosimilars are interchangeable with their reference products or equivalent biosimilars. Healthcare systems in Europe are primarily publicly funded, with scope for payer-driven policies to boost the adoption of biosimilars and contain healthcare expenditures.
Biosimilars in Oncology Market Analysis by Regions, 2023 (%)
Buy the Full Report for More Regional Insights into the Biosimilars in Oncology Theme
Biosimilars in Oncology – Competitive Landscape
A few of the key companies offering biosimilars in the oncology sector are Sandoz, Celltrion, Amgen, Biocon, Pfizer, and Organon, among others.
Sandoz: Sandoz is leading in developing, manufacturing, and commercializing of generic and biosimilar medicines. The company offers products to treat cancer, cardiovascular, dermatological, neurological, ophthalmic, respiratory, and infectious diseases, as well as immune disorders. Sandoz was the first company to own an approved biosimilar and has established itself as a main player since then.
Amgen: Amgen Inc. discovers, develops, manufactures, and markets novel human medicines in six focused disease areas, namely cancer, inflammation, cardiovascular disease, nephrology, bone health, and neurological disorders. Amgen leverages its biologic manufacturing expertise to develop biosimilars and has launched often-prescribed biosimilars in oncology.
Biosimilars in Oncology Market Analysis by Companies
Buy the Full Report for More Company Insights into the Biosimilars in Oncology Theme
Key Highlights
Report deliverables include a PowerPoint report
Survey & Clinical trial analysis includes 8 countries
4 KOLs and one payer interviewed
Bristol-Myers Squibb Co
Sanofi
Amgen Inc
Sandoz
Celltrion
Biocon
Pfizer
Organon
Regeneron
Merck & Co
Table of Contents
Frequently asked questions
-
What was the biosimilar market size in the 8MM in 2022?
The biosimilar market size in the 8MM was $4 billion in 2022.
-
What is the expected growth rate of the biosimilar market during the forecast period?
The biosimilar market is expected to achieve a CAGR of more than 9% during 2022-2029.
-
Which region accounted for the highest biosimilar adoption rate in 2023?
Europe accounted for the highest adoption of biosimilars globally.
-
Which are the key companies associated with the biosimilars in oncology theme?
A few of the key companies associated with biosimilars in oncology are Sandoz, Celltrion, Amgen, Biocon, Pfizer, and Organon, among others.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Oncology reports