Cardiac Rhythm Management Devices Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Cardiac Rhythm Management Devices Pipeline Market Report Overview
Cardiac Rhythm Management (CRM) devices are those devices which are used to restore the natural synchronized pumping rhythm of the heart. These include Pacemakers, Cardiac Resynchronisation Therapy (CRT), Implantable Loop Recorders and Implantable Cardioverter Defibrillators (ICD). These devices are used for the treatment of congestive heart failure.
The Cardiac Rhythm Management Devices pipeline market research report provides comprehensive information about the Cardiac Rhythm Management Devices pipeline products with comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
Cardiac Rhythm Management Devices Pipeline Market Segments
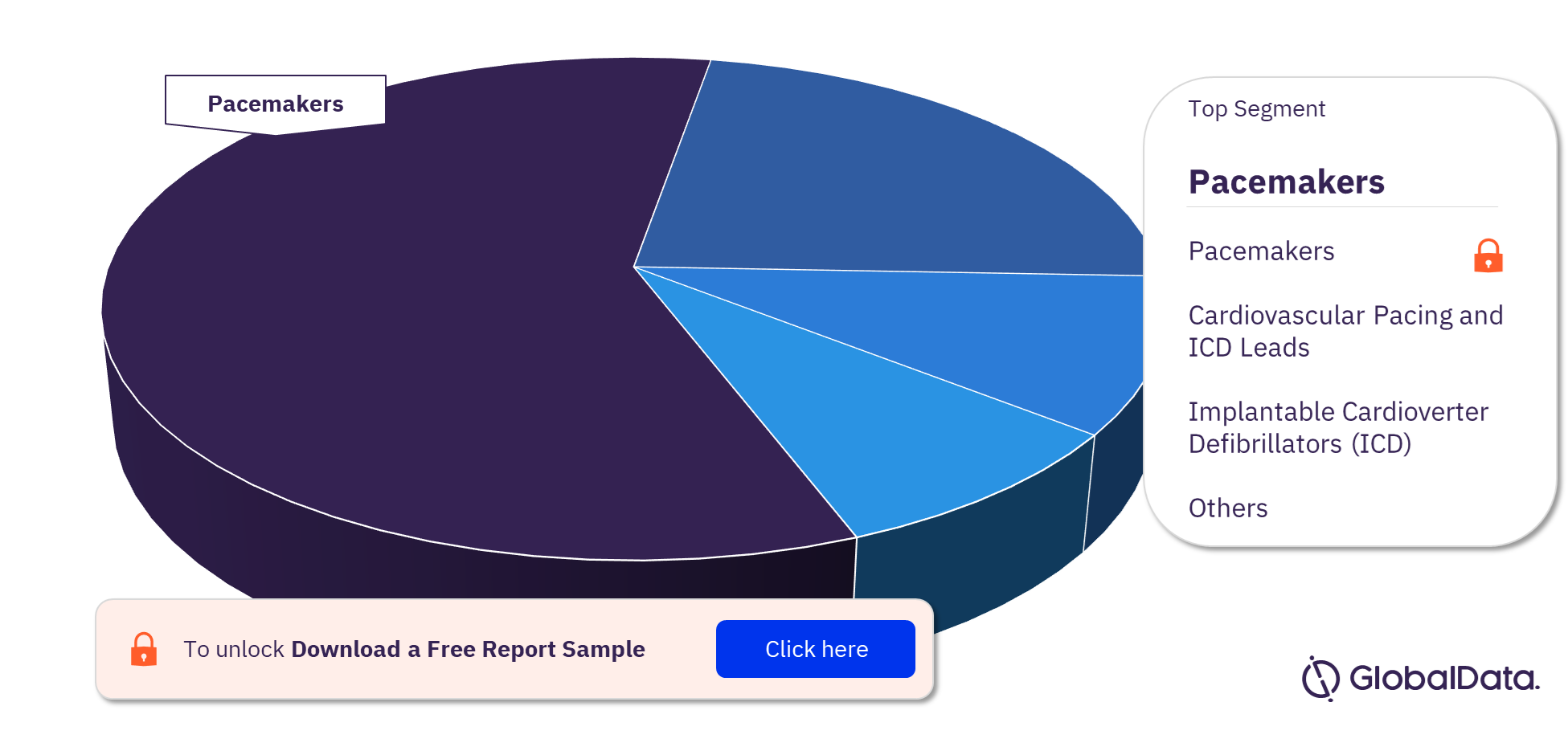
Some of the segments in the Cardiac Rhythm Management Devices pipeline market are Pacemakers, Cardiovascular Pacing and ICD Leads, Implantable Cardioverter Defibrillators (ICD), Cardiac Resynchronisation Therapy – Defibrillators (CRT-D), Cardiac Resynchronisation Therapy (CRT), Dual Chamber Pacemakers, Implantable Loop Recorders (ILR), Cardiac Resynchronisation Therapy – Pacemakers (CRT-P), Single Chamber Pacemakers, and Single Chamber Pacemakers with Leads. As of April 2023, Pacemakers has the highest number of pipeline products.
Pacemaker: A pacemaker is a device designed to regulate the beating of the heart by providing electrical impulses delivered by the electrodes contacting the heart muscle. It can be a single chamber or dual chamber type.
Implantable Loop Recorders (ILR): An Implantable loop recorder (ILR) is a subcutaneous, single-lead, electrocardiographic (ECG) monitoring device which is used for diagnosis in patients with recurrent unexplained episodes of palpitations or syncope. It is also useful for long-term monitoring in patients at risk for or with atrial fibrillation, structural heart disease, bundle branch block, recurrent neurally mediated syncope etc. The device consists of an insertable recorder that comes with a battery and two surface electrodes.
Cardiac Rhythm Management Devices Pipeline Market Analysis, by Segments, 2023 (%)
For more segment insights into the Cardiac Rhythm Management Devices pipeline market, download a free report sample
Cardiac Rhythm Management Devices Pipeline Market Segmentation by Territories
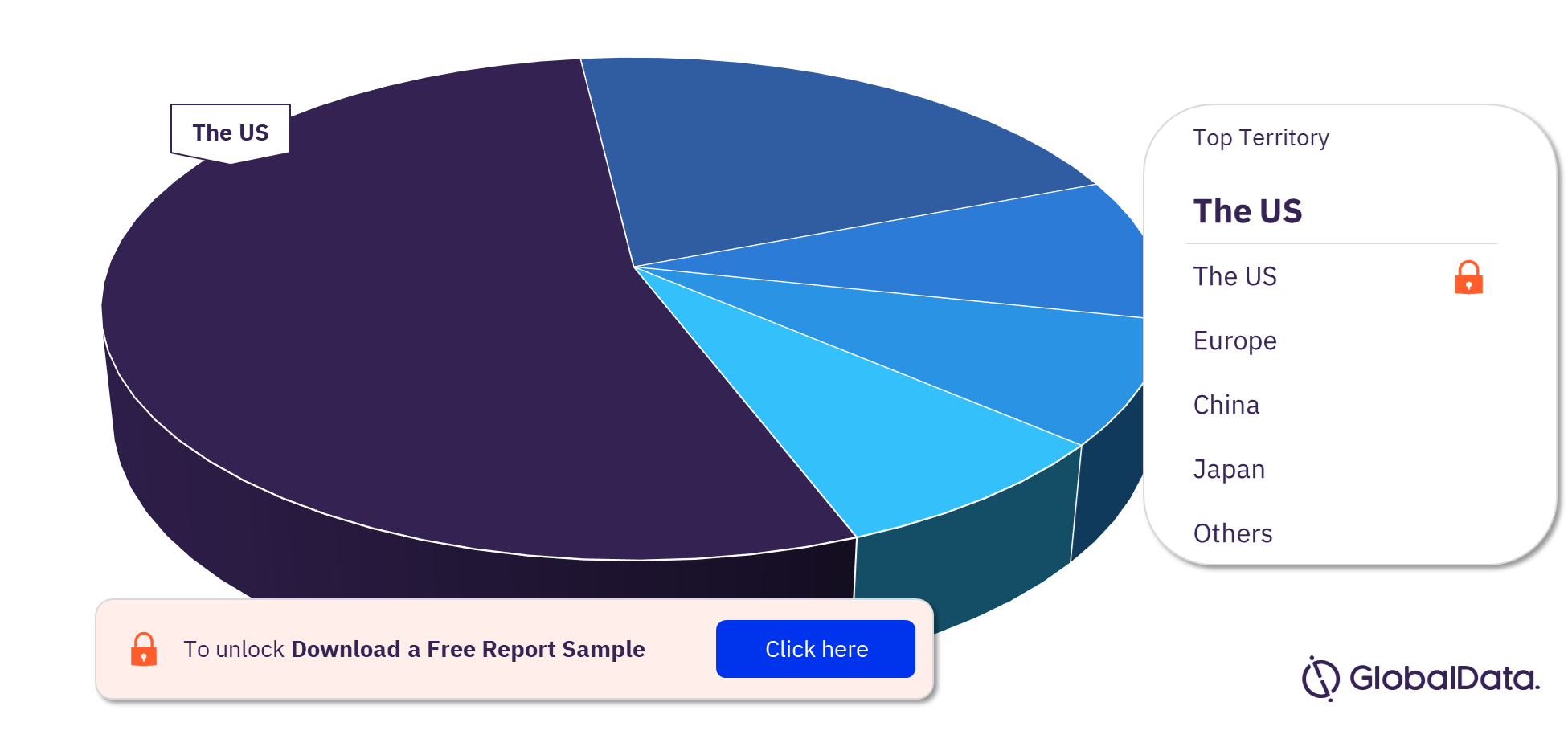
Some of the territories in the Cardiac Rhythm Management Devices pipeline market are the US, Europe, China, Japan, Israel, Australia, India, New Zealand, South Korea, and Taiwan. As of April 2023, the US has the highest number of pipeline products.
Cardiac Rhythm Management Devices Pipeline Market Analysis, by Territories, 2023 (%)
For more territory insights into the Cardiac Rhythm Management Devices pipeline market, download a free report sample
Cardiac Rhythm Management Devices Pipeline Market Segmentation by Regulatory Paths
Some of the regulatory paths in the Cardiac Rhythm Management Devices pipeline market are PMA, CE, NMPA, Shonin, TGA, Ninsho, 510(k), ICAC, MDL, and NIFDS. As of April 2023, PMA is the most followed pathway for pipeline products.
Cardiac Rhythm Management Devices Pipeline Market Analysis, by Regulatory Paths, 2023 (%)
For more regulatory path insights into the Cardiac Rhythm Management Devices pipeline market, download a free report sample
Cardiac Rhythm Management Devices Pipeline Market – Competitive Landscape
Some of the key companies in the Cardiac Rhythm Management Devices pipeline market are A3 Remote Monitoring Technologies Pvt Ltd, Advanced BioInformatics GmbH, AIS Inc., APT Medical Inc, Arizona State University, Arrhythmia Dynamics LLC, AtaCor Medical Inc, Bionet Sonar Inc, Biotronik SE & Co KG, and Boston Scientific Corp.
Arizona State University: Headquartered in Tempe, Arizona, the US, Arizona State University (ASU) is an educational and research university that provides entrepreneurship solutions. The university’s services include academic services, institutes and initiative consulting, industry engagement, knowledge enterprise development, support and consulting, global development, entrepreneurship, technology transfer, and undergraduate opportunity services. Its academic services comprise of degree programs, college and school programs, academic calendars, libraries, online degree, registration and scholarships, and financial aid services.
Cardiac Rhythm Management Devices Pipeline Market Report Overview
| Key Territories | The US, Europe, China, Japan, Israel, Australia, India, New Zealand, South Korea, and Taiwan |
| Key Segments | Pacemakers, Cardiovascular Pacing and ICD Leads, Implantable Cardioverter Defibrillators (ICD), Cardiac Resynchronisation Therapy – Defibrillators (CRT-D), Cardiac Resynchronisation Therapy (CRT), Dual Chamber Pacemakers, Implantable Loop Recorders (ILR), Cardiac Resynchronisation Therapy – Pacemakers (CRT-P), Single Chamber Pacemakers, and Single Chamber Pacemakers with Leads |
| Key Regulatory Paths | PMA, CE, NMPA, Shonin, TGA, Ninsho, 510(k), ICAC, MDL, and NIFDS |
| Leading Companies | A3 Remote Monitoring Technologies Pvt Ltd, Advanced BioInformatics GmbH, AIS Inc., APT Medical Inc, Arizona State University, Arrhythmia Dynamics LLC, AtaCor Medical Inc, Bionet Sonar Inc, Biotronik SE & Co KG, and Boston Scientific Corp |
Segments Covered in the Report
Cardiac Rhythm Management Devices Pipeline Market Segments Outlook
- Pacemakers
- Cardiovascular Pacing and ICD Leads
- Implantable Cardioverter Defibrillators (ICD)
- Cardiac Resynchronisation Therapy – Defibrillators (CRT-D)
- Cardiac Resynchronisation Therapy (CRT)
- Dual Chamber Pacemakers
- Implantable Loop Recorders (ILR)
- Cardiac Resynchronisation Therapy – Pacemakers (CRT-P)
- Single Chamber Pacemakers
- Single Chamber Pacemakers with Leads
- Subcutaneous Implantable Cardioverter Defibrillators (ICD)
- Single Chamber Implantable Cardioverter Defibrillators (ICD)
- Dual Chamber Leadless Pacemakers
- Single Chamber Leadless Pacemakers
- Cardiac Rhythm Management Devices Pipeline Market Territories Outlook
- Cardiac Rhythm Management Devices Pipeline Market Regulatory Paths Outlook
Cardiac Rhythm Management Devices Pipeline Market Territories Outlook
- The US
- Europe
- China
- Japan
- Israel
- Australia
- India
- New Zealand
- South Korea
- Taiwan
- United Kingdom
- Canada
- Bangladesh
- Costa Rica
- El Salvador
- Singapore
- Mexico
Cardiac Rhythm Management Devices Pipeline Market Regulatory Paths Outlook
- PMA
- CE
- NMPA
- Shonin
- TGA
- Ninsho
- 510(k)
- ICAC
- MDL
- NIFDS
- BOPA
- HDE Approvals
- UKCA
- HSA
Scope
- Extensive coverage of the Cardiac Rhythm Management Devices under development
- Reviews details of major pipeline products which includes, product description, licensing and collaboration details and other developmental activities
- Reviews the major players involved in the development of Cardiac Rhythm Management Devices and list all their pipeline projects
- The coverage of pipeline products based on various stages of development ranging from Early Development to Approved / Issued stage
- The report provides key clinical trial data of ongoing trials specific to pipeline products
- Recent developments in the segment / industry
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies
- Identify emerging players with potentially strong product portfolio and create effective counter strategies to gain competitive advantage
- Identify and understand important and diverse types of Cardiac Rhythm Management Devices under development
- Develop market-entry and market expansion strategies
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline
- In-depth analysis of the product’s current stage of development, territory and estimated launch date
Advanced BioInformatics GmbH
AIS Inc.
APT Medical Inc
Arizona State University
Arrhythmia Dynamics LLC
AtaCor Medical Inc
Bionet Sonar Inc
Biotronik SE & Co KG
Boston Scientific Corp
Boston TransTec LLC
Cairdac
CardiaLen, Inc.
Cardioalarm, Inc
Ceryx Medical Ltd
Children’s National Health System
City Labs Inc
Corhythm Inc
Coridea, LLC
CVRx Inc
DHS Medical
Drexel University
Duke University
EBR Systems Inc
Endomimetics LLC
Endurance Rhythm Inc.
Hadassah Medical Center
Impulse Dynamics Germany GmbH
InnerPulse Inc (Inactive)
Integer Holdings Corp
IRadimed Corp
Johns Hopkins University
Kenergy Inc.
Krisara Engineering, LLC
Massachusetts General Hospital
Max Planck Institute for Dynamics and Self-Organization
Mayo Clinic
Medicool Technologies Inc
Medtronic Inc
Medtronic Plc
MicroPort CRM SA
MicroPort Scientific Corp
NanoLinea
NewPace Ltd
Northwestern University
NuVascular Technologies Inc
Odem Medical Ltd
Ohio State University
Osypka Medical GmbH
Perpetuum Ltd
Queen Mary University of London
Rice University
Shaanxi Qinming Medical Instruments Co Ltd
Smartwave Medical Ltd (Inactive)
Spectranetics Corp
St. Jude Medical LLC
SUNY Downstate Medical Center
Suzhou Singular Medical Co Ltd
Teleflex Inc
Thayer School of Engineering at Dartmouth
U.S. Stem Cell Inc
University of Arizona
University of Bern
University of California Los Angeles
University of Chicago
University of Glasgow
University of Michigan
University of Southern California
University of Utah
Table of Contents
Table
Figures
Frequently asked questions
-
Which territory has the highest number of pipeline products in the Cardiac Rhythm Management Devices pipeline market as of April 2023?
As of April 2023, the US has the highest number of pipeline products in the Cardiac Rhythm Management Devices pipeline market.
-
Which segment accounted for the largest Cardiac Rhythm Management Devices pipeline market share?
Pacemakers accounted for the largest Cardiac Rhythm Management Devices pipeline market share.
-
Which is the most followed regulatory pathway in the Cardiac Rhythm Management Devices pipeline market As of April 2023?
As of April 2023, PMA is the most followed regulatory pathway in the Cardiac Rhythm Management Devices pipeline market.
-
Who are the major players operating in the Cardiac Rhythm Management Devices pipeline market?
Some of the major players operating in the Cardiac Rhythm Management Devices pipeline market are A3 Remote Monitoring Technologies Pvt Ltd, Advanced BioInformatics GmbH, AIS Inc., APT Medical Inc, Arizona State University, Arrhythmia Dynamics LLC, AtaCor Medical Inc, Bionet Sonar Inc, Biotronik SE & Co KG, and Boston Scientific Corp.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Cardiac Rhythm Management Devices reports