Likelihood of Approval and Phase Transition Success Rate Model – Cemiplimab in Natural Killer Cell Lymphomas
Powered by ![]()
Unlock hidden opportunities in the LoA industry
Empower your strategies with our Likelihood of Approval and Phase Transition Success Rate Model – Cemiplimab in Natural Killer Cell Lymphomas report and make more profitable business decisions.
This report provides you with the data that allows you to track and predict the specific likelihood of approval (LOA) and phase transition success rate (PTSR) of a drug using GlobalData’s proprietary machine learning algorithms developed using over 10 years of historical data.
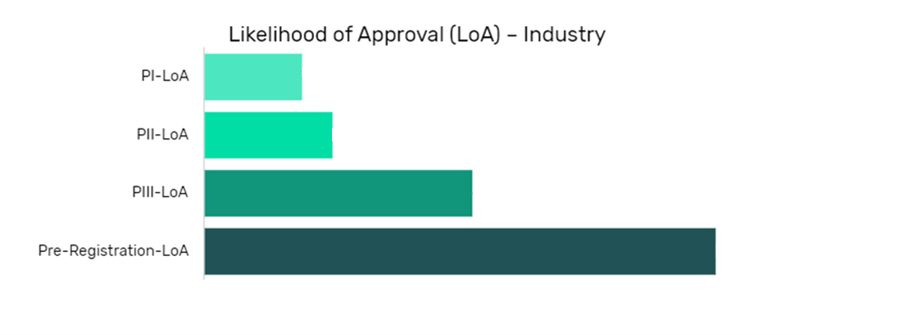
Cemiplimab in Natural Killer Cell Lymphomas Drug Details:
Cemiplimab (Libtayo) is an anti-neoplastic agent. It is formulated as concentrate solution for intravenous route of administration. Libtayo is indicated for the treatment of patients with metastatic squamous cell carcinoma (SCC) or locally advanced squamous cell carcinoma who are not candidates for curative surgery or curative radiation. Libtayo is indicated for the treatment of patients with locally advanced basal cell carcinoma (laBCC) previously treated with a hedgehog pathway inhibitor or for whom a hedgehog pathway inhibitor is not appropriate and in patients with metastatic basal cell carcinoma (mBCC) previously treated with a hedgehog pathway inhibitor or for whom a hedgehog pathway inhibitor is not appropriate. Libtayo is indicated for the first-line treatment of patients with non-small cell lung cancer (NSCLC) whose tumors have high PD-L1 expression [Tumor Proportion Score (TPS) = 50%] as determined by an FDA-approved test, with no EGFR, ALK or ROS1 aberrations, and is locally advanced where patients are not candidates for surgical resection or definitive chemoradiation or metastatic.Cemiplimab (REGN-2810) is under development for the treatment of merkel cell carcinoma (MCC), advanced/metastatic penile carcinoma, recurrent glioblastoma multiforme (GBM), cutaneous squamous cell carcinoma, advanced solid tumors including metastatic pancreatic cancer, epithelial ovarian cancer, primary peritoneal, advanced or metastatic secondary angiosarcoma, metastatic adenocarcinoma of the pancreas, metastatic hepatocellular carcinoma, fallopian tube cancer, thyroid cancer, colon cancer colorectal cancer, metastatic prostate cancer (as a first-line therapy), metastatic hormone-sensitive prostate cancer, metastatic non-squamous non-small cell lung cancer (NSCLC), relapsed or refractory multiple myeloma, pediatric glioblastoma, advanced malignancies, newly diagnosed glioblastoma, melanoma, basal cell carcinoma, non-small cell lung cancer, cutaneous squamous cell carcinoma, relapsed or refractory natural killer cell lymphoma, T-cell lymphomas, metastatic castration-resistant prostate cancer, colorectal cancer, triple negative breast cancer and adenocarcinoma of the prostate, cervical cancer, HPV16 positive oropharyngeal cancer, clear cell renal cell carcinoma and advanced hematologic malignancies including relapsed and refractory B-cell Hodgkin lymphoma, B-cell non-Hodgkin lymphoma, head and neck squamous cell carcinoma, breast cancer (adjuvant), oral cancer, nasopharyngeal cancer, hypopharyngeal cancer, metastatic uveal melanoma and acute lymphoblastic leukemia. It is administered intravenously and intralesionally. The drug candidate is a fully human hinge-stabilized IgG4P monoclonal antibody which acts by targeting the PD-1 receptor. It is developed based on VelocImmune platform technology.It was also under development for the treatment of squamous non-small cell lung cancer, hepatitis B, newly diagnosed diffuse intrinsic pontine glioma in children, newly diagnosed or recurrent high-grade glioma in children.
Report Coverage
The data is segmented by drug name per indication and shows the current likelihood of approval for the drug compared to the indication benchmark and the industry benchmark.
The Likelihood of Approval data is updated regularly based on events that take place which impact the clinical development process and regulatory considerations. GlobalData’s proprietary machine learning models consider these events in real time, to produce quantitative changes to the LOA and PTSR along with qualitative reasoning why the likelihood of approval has changed.
| Quick View – Cemiplimab LOA Data | |||||
| Report Segments |
|
||||
| Drug Name |
|
||||
| Administration Pathway |
|
||||
| Therapeutic Areas |
|
||||
| Key Manufacturers |
|
||||
| Drug Development Status |
|
||||
Reasons to Buy
- Precise Likelihood of Approval and Phase Transition Success Rates: Our machine learning and proprietary models provide accurate predictions, helping you gauge the potential success of a drug in the regulatory process.
- Competitive Strategy Planning: Access information on LOA and PTSR for competitors’ drugs, allowing you to plan your clinical development, commercialisation and marketing strategies
- Event-driven Updates: Track event-driven changes in LOA and PTSR benchmarked against indication LOA/PTSR. Get the latest insights to adapt your strategies promptly!
- Well-informed Investment Decisions: This data helps you navigate the dynamic landscape of drug development and regulatory considerations.
Scope
- Drug Details: Drug name, Drug type, Intervention type
- Administration Pathway
- Therapeutic Areas
- Key Manufacturers
- Drug Development Status
This is an on-demand report that will be delivered upon request. The report will be delivered within 2 business days of the purchase, excluding weekends and holidays. Certain sections of the report may be removed or altered based on data availability and relevance.
Frequently asked questions
- Drugs which have been approved in the past 10 years
- Drugs which have failed during clinical development in the past 18 years
- Drugs which are currently in development
- Phase I, Phase II, Phase III, and Pre-Registration development stage
- Drugs must meet one of the following criteria to be included in the model:
- The developer has specified the US as an intended market for approval.
- The developer has not specified any country as an intended market for approval, i.e. the “Drug Geography” is listed as “Global”
- Innovator drugs and biosimilars
- Diagnostics, Imaging Agents, Biomarkers, stents and other drug delivery devices (covered in GlobalData’s Medical Intelligence Center).
- Nutraceuticals, dietary supplements, alternative medicines, imaging agents, radio emitter, transplants, transfusions, fillers, cosmetics, probiotics, antiseptics, antacids, mobilizing agents, veterinary drugs and drugs not seeking approval.
- Generic drugs
- Innovative drugs in Preclinical or Discovery Stage.
- Pipeline drugs sponsored by a Government or Institution.
- Drugs with a specific Drug Geography not the United States.
The probability of a drug ultimately receiving market authorization
The probability of a drug’s advancement to the next stage of clinical development
GlobalData’s Drug-Specific Likelihood of Approval (LoA) calculates the Phase Transition Success Rate (PTSR) and Likelihood of Approval (LoA) customized to individual drug. The model uses a combination of Machine Learning (ML) and a GlobalData proprietary algorithm to process data points from the Drugs, Clinical Trials, Regulatory Milestones, Company, and Financial databases.
Inclusion
Data Scope:
Drug Phase Scope:
Drug Geography Scope:
Drug Type Scope:
Entity Type Scope:
Only drugs in development by companies are included in the model.
Exclusion
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Related reports
View more Pharmaceuticals reports







