Critical Care Ventilators Pipeline by Development Stages, Segments, Region and Countries, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Critical Care Ventilators Pipeline Market Report Overview
Critical care ventilators are also known as intensive care ventilators. They provide artificial respiratory support to critical patients in ICUs. The critical care ventilators pipeline market research report provides a comprehensive understanding of the pipeline products along with their comparative analysis at various stages of development. The report also includes information about the territories wherein the clinical trials are in progress, the regulatory paths followed by the trials, and the companies associated with the trials.
| Key Territories | · The US
· India · Europe · The UK · Malaysia |
| Key Regulatory Paths | · EUA
· ICAC · 510(k) · CE |
| Leading Companies | · AgVa Healthcare Pvt Ltd
· Ashok Leyland Ltd · Babcock International Group Plc · Ben-Gurion University of the Negev · Bessel LLC |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
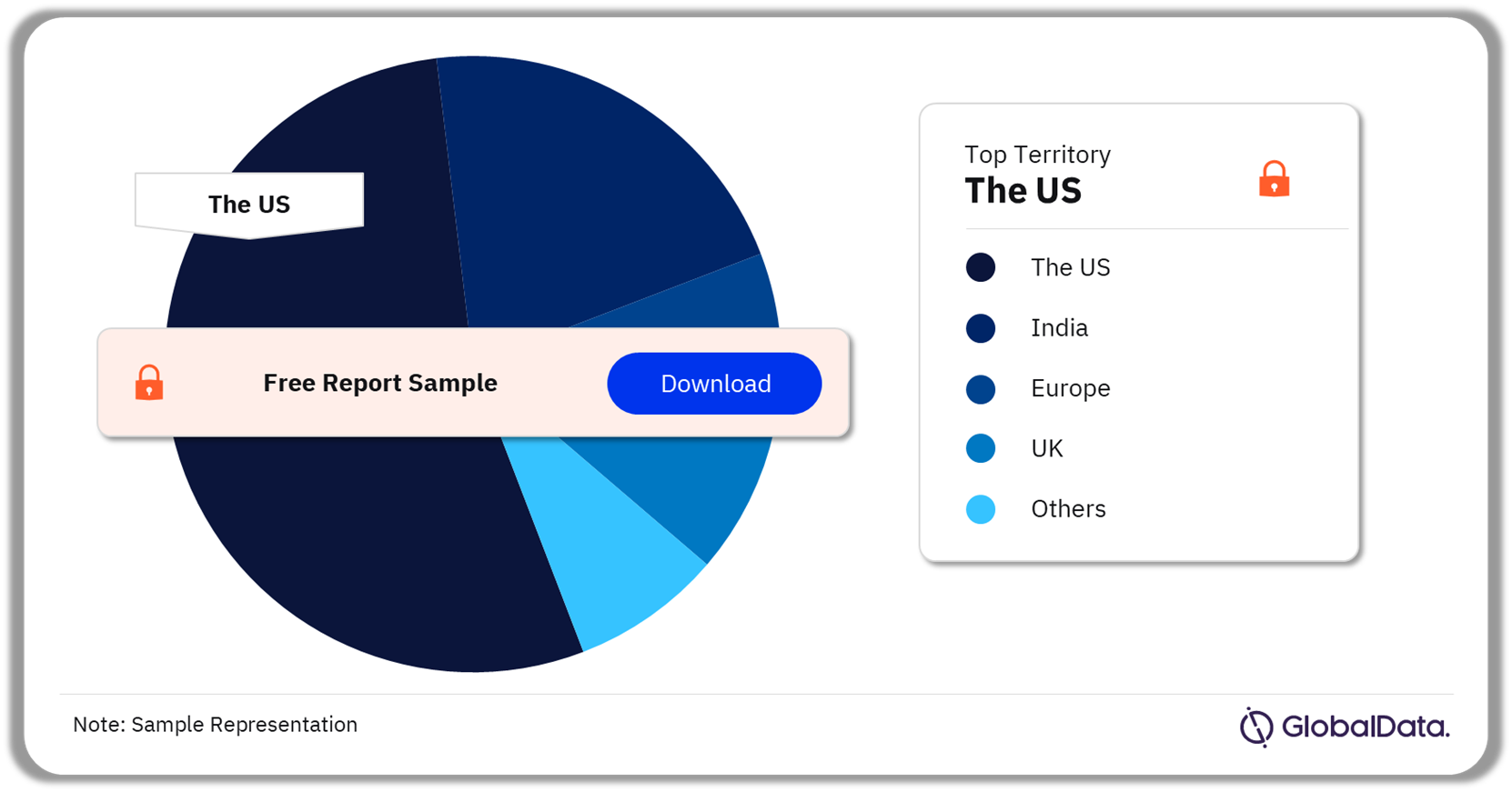
Critical Care Ventilators Pipeline Market Segmentation by Territories
A few of the key territories for the critical care ventilators pipeline market are the US, India, Europe, the UK, and Malaysia. As of February 2024, the US accounted for the highest number of pipeline products.
Critical Care Ventilators Pipeline Market Analysis by Territories, 2024 (%)
Buy the Full Report for More Territory Insights into the Critical Care Ventilators Pipeline Market
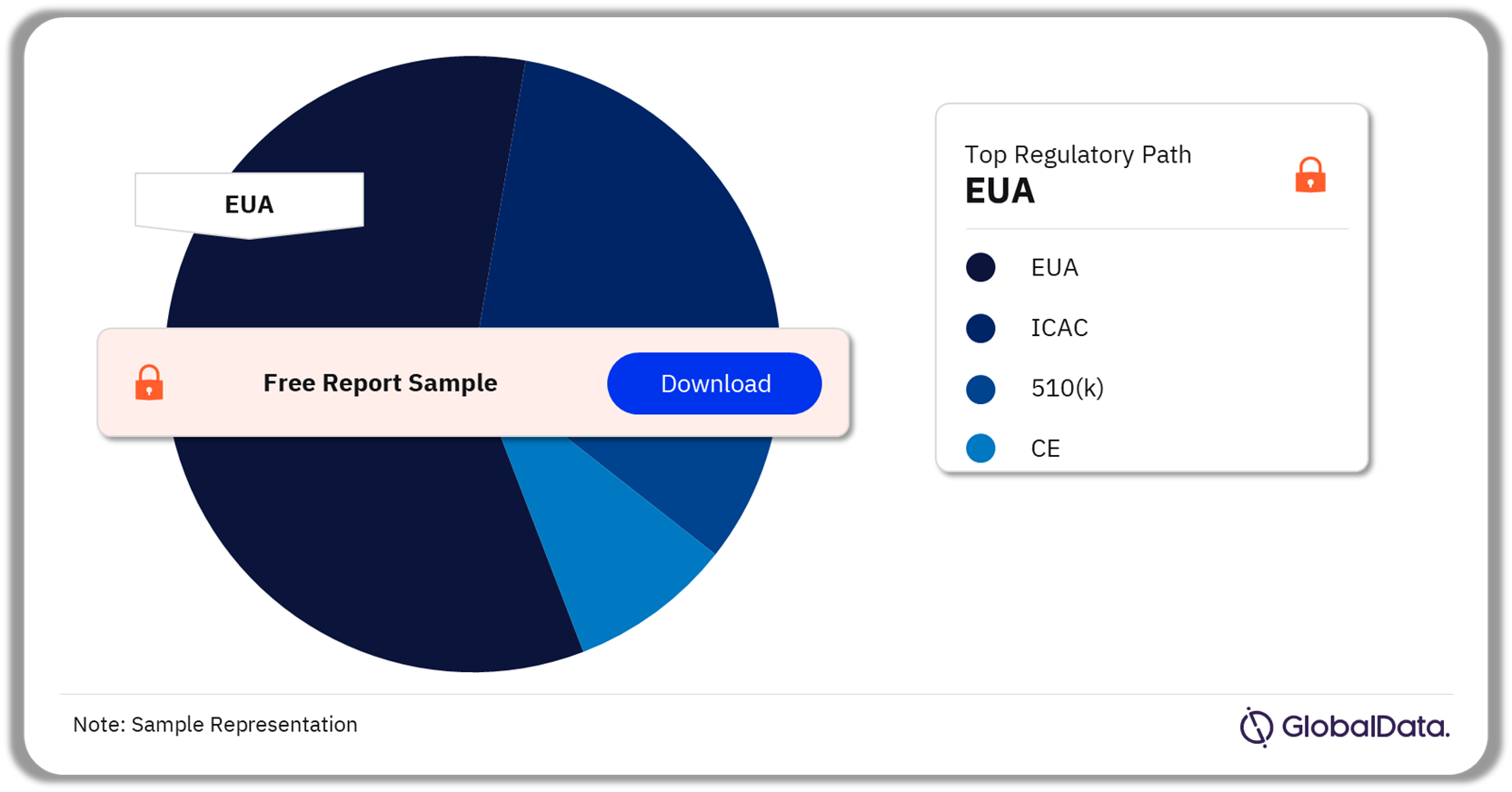
Critical Care Ventilators Pipeline Market Segmentation by Regulatory Paths
The key regulatory paths in the critical care ventilators pipeline products market are EUA, ICAC, 510(k), and CE. As of February 2024, EUA was the most followed pathway for pipeline products in the market.
Critical Care Ventilators Pipeline Market Analysis by Regulatory Paths, 2024 (%)
Buy the Full Report for More Regulatory Path Insights into the Critical Care Ventilators Pipeline Market
Critical Care Ventilators Pipeline Market – Competitive Landscape
A few of the key companies associated with the critical care ventilators pipeline market are AgVa Healthcare Pvt Ltd, Ashok Leyland Ltd, Babcock International Group Plc, Ben-Gurion University of the Negev, and Bessel LLC, among others.
Ashok Leyland Ltd: Ashok Leyland Ltd is headquartered in Chennai, Tamil Nadu, India. It is an integrated automobile company that offers heavy and medium commercial vehicles and related components for light commercial vehicles, along with power solution systems. The company has joint venture partnerships with Alteams Group for the manufacturing of high-press die-casting extruded aluminum components for the automotive and telecommunications sectors, and John Deere for construction equipment.
Babcock International Group Plc: Babcock is headquartered in London, Greater London, the UK. The company provides engineering support services. It offers naval support services, technical services, testing, assembly, consultancy, and training for the marine sector. The company provides vehicle fleet management services, equipment support, and technical training for military and civil customers. It also offers lifesaving search and rescue flights, emergency environmental operations, emergency medical services, integrated military engineering, training and facilities services, and critical crew change services in the North Sea.
Critical Care Ventilators Pipeline Market Analysis by Companies, 2024
Buy the Full Report for More Company Insights into the Critical Care Ventilators Pipeline Market
Segments Covered in the Report
Critical Care Ventilators Pipeline Market Territories Outlook
- The US
- India
- Europe
- The UK
- Malaysia
Critical Care Ventilators Pipeline Market Regulatory Paths Outlook
- EUA
- ICAC
- 510(k)
- CE
Scope
This report provides:
- Extensive coverage of the critical care ventilators under development.
- Exhaustive list of major pipeline products and their details, including product description, licensing and collaboration details, and other developmental activities.
- Reviews of major players involved in the development of critical care ventilators and lists all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from early development to approved/issued stage.
- Key clinical trial data of ongoing clinical trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with potentially strong product portfolios and create effective counter strategies to gain a competitive advantage.
- Identify and understand important and diverse types of Critical Care Ventilators under development.
- Develop market-entry and market-expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- Carry out an in-depth analysis of the product’s current stage of development, territory, and estimated launch date.
Ashok Leyland Ltd
Babcock International Group Plc
Ben-Gurion University of the Negev
Bessel LLC
Bhagwati Products Ltd
Cambridge Consultants Ltd
Carlos III University of Madrid
ConzumeX Industries Pvt Ltd
Ezz Medical Industries Co
Gas N2itrogen SL
Georgia Institute of Technology
Gilero LLC
Hamilton Medical AG
Imperial College London
Inali Foundation
Inbentus
Indian Institute of Science
Indian Institute of Technology Roorkee
Integrated Polytechnic Regional College Kigali
Khalifa University
Kiira Motors Corp
Kritikare India Pvt Ltd
Lawrence Livermore National Laboratory
Mahindra & Mahindra Ltd
Massachusetts Institute of Technology
Naval Sea Logistics Center
OneBreath Inc.
Quantaira Health
Rathinam Group of Institutions
SLE Ltd
Sree Chitra Tirunal Institute for Medical Sciences & Technology
Steros GPA Innovative SL
Stogger BV
The Chaim Sheba Medical Center
Tolomatic Inc
Universiti Teknologi MARA
University of Antioquia
University of Arizona
University of California Los Angeles
University of Connecticut
University of Florida
Wise Ally International Holdings Ltd
X-Biomedical Inc
Table of Contents
Table
Figures
Frequently asked questions
-
Which territory had the highest number of products in the critical care ventilators pipeline market?
As of February 2024, the US accounted for the highest number of pipeline products in the critical care ventilators market.
-
Which was the most followed regulatory pathway in the critical care ventilators pipeline market?
As of February 2024, EUA was the most followed pathway for pipeline products in the critical care ventilators market.
-
Which are the major companies operating in the critical care ventilators pipeline market?
A few of the key companies associated with the critical care ventilators pipeline market are AgVa Healthcare Pvt Ltd, Ashok Leyland Ltd, Babcock International Group Plc, Ben-Gurion University of the Negev, and Bessel LLC, among others.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Critical Care Ventilators reports