Crohn’s Disease (Regional Enteritis) Drugs in Development by Stages, Target, MoA, RoA, Molecule Type and Key Players, 2022 Update
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Crohns Disease Pipeline Drugs Market Report Overview
Crohn’s Disease (Regional Enteritis) pipeline market research report provides comprehensive information on the therapeutics under development for Crohn’s Disease (Regional Enteritis), complete with analysis by stage of development, drug target, mechanism of action (MoA), route of administration (RoA) and molecule type. The report also covers the descriptive pharmacological action of the therapeutics, its complete research and development history and latest news and press releases. Additionally, the report provides an overview of key players involved in therapeutic development for Crohn’s Disease (Regional Enteritis) and features dormant and discontinued projects.
Key Targets in the Regional Enteritis Pipeline Market
The key targets in the regional enteritis pipeline market are Interleukin 23 Subunit Alpha, Tumor Necrosis Factor, Interleukin 12 Subunit Beta, Integrin Alpha 4, Tyrosine Protein Kinase JAK1, Cannabinoid Receptor 1, and Cannabinoid Receptor 2. Interleukin 23 Subunit Alpha has the highest number of pipeline products.
Regional Enteritis Pipeline Market, by Targets
For more target insights, download a free report sample
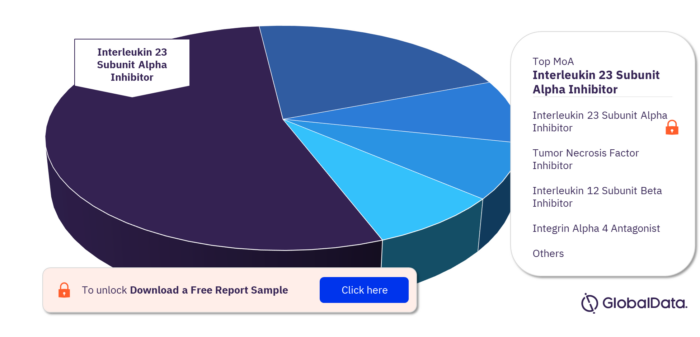
Key MoA in the Regional Enteritis Pipeline Market
The key MoA in the regional enteritis pipeline market are Interleukin 23 Subunit Alpha Inhibitor, Tumor Necrosis Factor Inhibitor, Interleukin 12 Subunit Beta Inhibitor, Integrin Alpha 4 Antagonist, Tyrosine Protein Kinase JAK1 Inhibitor, and Cannabinoid Receptor 1 Agonist. Interleukin 23 Subunit Alpha Inhibitor has the highest number of pipeline products.
Regional Enteritis Pipeline Market, by MoA
For more MoA insights, download a free report sample
Key RoA in the Regional Enteritis Pipeline Market
The key RoA in the regional enteritis pipeline market are oral, subcutaneous, intravenous, topical, rectal, nasal, and parenteral. Oral has the highest number of pipeline products.
Regional Enteritis Pipeline Market, by RoA
For more RoA insights, download a free report sample
Key Molecule Types in the Regional Enteritis Pipeline Market
The key molecule types in the regional enteritis pipeline market are small molecule, monoclonal antibody, biologic, cell therapy, recombinant protein, polysaccharide, and gene-modified cell therapy. Small molecule has the highest number of pipeline products.
Regional Enteritis Pipeline Market, by Molecule Types
For more molecule type insights, download a free report sample
Key Companies in the Regional Enteritis Pipeline Market
The key companies in the regional enteritis pipeline market are AbbVie Inc, Johnson & Johnson, Dadang & BIO Co Ltd, Boehringer Ingelheim International GmbH, Landos Biopharma Inc, Takeda Pharmaceutical Co Ltd, and Bristol-Myers Squibb Co. AbbVie Inc has the highest number of pipeline products.
Regional Enteritis Pipeline Market, by Companies
To know more about companies, download a free report sample
Market report overview
| Key targets | Interleukin 23 Subunit Alpha, Tumor Necrosis Factor, Interleukin 12 Subunit Beta, Integrin Alpha 4, Tyrosine Protein Kinase JAK1, Cannabinoid Receptor 1, and Cannabinoid Receptor 2 |
| Key MoA | Interleukin 23 Subunit Alpha Inhibitor, Tumor Necrosis Factor Inhibitor, Interleukin 12 Subunit Beta Inhibitor, Integrin Alpha 4 Antagonist, Tyrosine Protein Kinase JAK1 Inhibitor, and Cannabinoid Receptor 1 Agonist |
| Key RoA | Oral, Subcutaneous, Intravenous, Topical, Rectal, Nasal, and Parenteral |
| Key molecule types | Small Molecule, Monoclonal Antibody, Biologic, Cell Therapy, Recombinant Protein, Polysaccharide, and Gene-Modified Cell Therapy |
| Key companies | AbbVie Inc, Johnson & Johnson, Dadang & BIO Co Ltd, Boehringer Ingelheim International GmbH, Landos Biopharma Inc, Takeda Pharmaceutical Co Ltd, and Bristol-Myers Squibb Co |
Scope
- The pipeline guide provides a snapshot of the global therapeutic landscape of Crohn’s Disease (Gastrointestinal).
- The pipeline guide reviews pipeline therapeutics for Crohn’s Disease (Gastrointestinal) by companies and universities/research institutes based on information derived from company and industry-specific sources.
- The pipeline guide covers pipeline products based on several stages of development ranging from pre-registration till discovery and undisclosed stages.
- The pipeline guide features descriptive drug profiles for the pipeline products which comprise, product description, descriptive licensing and collaboration details, R&D brief, MoA & other developmental activities.
- The pipeline guide reviews key companies involved in Crohn’s Disease (Gastrointestinal) therapeutics and enlists all their major and minor projects.
- The pipeline guide evaluates Crohn’s Disease (Gastrointestinal) therapeutics based on mechanism of action (MoA), drug target, route of administration (RoA) and molecule type.
- The pipeline guide encapsulates all the dormant and discontinued pipeline projects.
- The pipeline guide reviews latest news related to pipeline therapeutics for Crohn’s Disease (Gastrointestinal)
Reasons to Buy
- Procure strategically important competitor information, analysis, and insights to formulate effective R&D strategies.
- Recognize emerging players with potentially strong product portfolio and create effective counter-strategies to gain competitive advantage.
- Find and recognize significant and varied types of therapeutics under development for Crohn’s Disease (Gastrointestinal).
- Classify potential new clients or partners in the target demographic.
- Develop tactical initiatives by understanding the focus areas of leading companies.
- Plan mergers and acquisitions meritoriously by identifying key players and it’s most promising pipeline therapeutics.
- Formulate corrective measures for pipeline projects by understanding Crohn’s Disease (Gastrointestinal) pipeline depth and focus of Indication therapeutics.
- Develop and design in-licensing and out-licensing strategies by identifying prospective partners with the most attractive projects to enhance and expand business potential and scope.
- Adjust the therapeutic portfolio by recognizing discontinued projects and understand from the know-how what drove them from pipeline.
9 Meters Biopharma Inc
AbbVie Inc
Abivax SA
Aclaris Therapeutics Inc
Active Biotech AB
Affilogic SAS
AgomAb Therapeutics NV
AIBIOS Co Ltd
Alfasigma SpA
Algernon Pharmaceuticals Inc
Alpha Cancer Technologies Inc
Alvotech ehf
Amide Technologies Inc
Amytrx Therapeutics Inc
Anterogen Co Ltd
Artelo Biosciences Inc
Artizan Biosciences Inc
Asdera LLC
Asieris Pharmaceuticals Co Ltd
Assembly Biosciences Inc
AstraZeneca Plc
Atlantic Healthcare Plc
Avalo Therapeutics Inc
Avesthagen Ltd
Avexegen Therapeutics Inc
Avobis Bio LLC
Avotres Inc
Bio-Thera Solutions Ltd
Biocon Ltd
Biogen Inc
Biohaven Pharmaceutical Holding Company Ltd
BioXpress Therapeutics SA
Boehringer Ingelheim International GmbH
Bristol-Myers Squibb Co
Cellivery Therapeutics Inc
CELLnLIFE Inc
Celltrion Inc
Cellvation Inc
Celularity Inc
ChemoCentryx Inc
Chong Kun Dang Pharmaceutical Corp
ChunLab Inc
Cloud Pharmaceuticals Inc
Curacle Co Ltd
CVasThera
Cytodyn Inc
Dadang & BIO Co Ltd
Daiichi Sankyo Co Ltd
Defensin Therapeutics ApS
Deka Biosciences Inc
Denali Therapeutics Inc
Direct Biologics LLC
DM Bio Ltd
DNX Biopharmaceuticals Inc
EA Pharma Co Ltd
Eden Biologics Inc
Educell doo
Eisai Co Ltd
Eli Lilly and Co
Enterome Bioscience SA
Enzo Biochem Inc
Exeliom Biosciences SAS
Ferring International Center SA
Finch Therapeutics Group Inc
First Wave BioPharma Inc
FYB 202 GmbH & Co KG
Galactica Biotech Ltd
Galapagos NV
Genentech USA Inc
Genor BioPharma Co Ltd
Giiant Pharma Inc
GlaxoSmithKline Plc
Gossamer Bio Inc
Gusto Global LLC
HAV Vaccines Ltd
Himuka AM Pharma Corp
Hoth Therapeutics Inc
Huaxia Source Cell Engineering Group Co Ltd
Iltoo Pharma
Immix BioPharma Inc
Immunic Inc
Immupharma Plc
InflammatoRx inc
Innovation Pharmaceuticals Inc
Innovative Pharmacology Research
Innovimmune Biotherapeutics Inc
Intract Pharma Ltd
Jiangsu Carephar Pharmaceutical Co Ltd
Jiangsu Hengrui Medicine Co Ltd
JiangSu Qyuns Therapeutics Co Ltd
Johnson & Johnson
Jyant Technologies Inc
Kangstem Biotech Co Ltd
Koutif Therapeutics LLC
Kyorin Pharmaceutical Co Ltd
Landos Biopharma Inc
Lumen Bioscience Inc
Machavert Pharmaceuticals LLC
MAKScientific LLC
Mesoblast Ltd
Metacrine Inc
Metagen Therapeutics Inc
MetiMedi Pharmaceuticals Co Ltd
MGC Pharmaceuticals Ltd
Mitsubishi Tanabe Pharma Corp
Morphic Therapeutic Inc
MRM Health NV
Mycenax Biotech Inc
NeuClone Pty Ltd
Novellus Therapeutics Ltd
Oncodesign SA
Orbsen Therapeutics Ltd
Orchard Therapeutics Plc
PanTheryx Inc
Par'Immune SAS
Paradigm Biopharmaceuticals Ltd
Parvus Therapeutics Inc
PeLeMed Co Ltd
Pfizer Inc
Pharmapraxis
Polpharma Biologics SA
Praeventix LLC
Prestige BioPharma Ltd
Prometheus Biosciences Inc
Puretech Health Plc
Qu Biologics Inc
Rebiotix Inc
Rebus Holdings Inc
RedHill Biopharma Ltd
Redx Pharma Plc
ReForm Biologics Inc
Regentys Corp
Reistone Biopharma Co Ltd
Reponex Pharmaceuticals AS
Saje Pharma LLC
Samsung Bioepis Co Ltd
Sangamo Therapeutics Inc
Saniona AB
Semorex Technologies Ltd
Servatus Ltd
Shenzhen Evergreen Therapeutics Co Ltd
Siam Bioscience Co Ltd
Soligenix Inc
Statera Biopharma Inc
Steel Therapeutics Inc
Stempeutics Research Pvt Ltd
STERO Biotechs Ltd
Sublimity Therapeutics HoldCo Ltd
Suono Bio Inc
Surrozen Inc
Suzhou Pharmavan Cancer Research Center Co Ltd
Synedgen Inc
Synlogic Inc
TaiwanJ Pharmaceuticals Co Ltd
Takeda Pharmaceutical Co Ltd
Temisis Therapeutics
Tetherex Pharmaceuticals Corp
The Cell Factory BVBA
Theravance Biopharma Inc
Thetis Pharmaceuticals LLC
Tiziana Life Sciences Plc
TRACT Therapeutics Inc
Trio Medicines Ltd
UCB SA
Ventyx Biosciences Inc
VHsquared Ltd
VITAbolus Inc
Vitro Diagnostics Inc
Whan In Pharm Co Ltd
Winston Pharmaceuticals Inc
XBiotech Inc
Yom Chai
YSOPIA Bioscience SA
Table of Contents
Table
Figures
Frequently asked questions
-
What are the key targets in the regional enteritis pipeline market?
The key targets in the regional enteritis pipeline market are Interleukin 23 Subunit Alpha, Tumor Necrosis Factor, Interleukin 12 Subunit Beta, Integrin Alpha 4, Tyrosine Protein Kinase JAK1, Cannabinoid Receptor 1, and Cannabinoid Receptor 2.
-
What are the key MoA in the regional enteritis pipeline market?
The key MoA in the regional enteritis pipeline market are Interleukin 23 Subunit Alpha Inhibitor, Tumor Necrosis Factor Inhibitor, Interleukin 12 Subunit Beta Inhibitor, Integrin Alpha 4 Antagonist, Tyrosine Protein Kinase JAK1 Inhibitor, and Cannabinoid Receptor 1 Agonist.
-
What are the key RoA in the regional enteritis pipeline market?
The key RoA in the regional enteritis pipeline market are oral, subcutaneous, intravenous, topical, rectal, nasal, and parenteral.
-
What are the key molecule types in the regional enteritis pipeline market?
The key molecule types in the regional enteritis pipeline market are small molecule, monoclonal antibody, biologic, cell therapy, recombinant protein, polysaccharide, and gene-modified cell therapy.
-
What are the key companies in the regional enteritis pipeline market?
The key companies in the regional enteritis pipeline market are AbbVie Inc, Johnson & Johnson, Dadang & BIO Co Ltd, Boehringer Ingelheim International GmbH, Landos Biopharma Inc, Takeda Pharmaceutical Co Ltd, and Bristol-Myers Squibb Co.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Gastrointestinal reports