Dental Implants and Abutments Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Dental Implants and Abutments Pipeline Market Report Overview
Dental implants are cylindrical and/or tapered posts that serve as a substitute for the tooth root and provide a strong and sturdy foundation for one or more replacement teeth. They are usually made of titanium or zirconia. Dental abutments are connecting elements between implants and the dental prosthesis. There is one final abutment per implant.
The Dental Implants and Abutments pipeline market research report provides comprehensive information about the dental implants and abutments pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials that are in progress.
| Key Segments | · Dental Implants
· Dental Abutments · Titanium Dental Implants · Zirconium Dental Implants · Internal Connectors |
| Key Territories | · The US
· Europe · China · Singapore · Canada · Argentina · Australia |
| Key Regulatory Paths | · 510(k)
· CE · HSA · NMPA · MDL · Ninsho · BOPA |
| Leading Companies | · AddBio AB
· BiteOn NV · Boston University · Coland Holdings Limited · Face Your Face HandelsGesmbH · Hefei Boyamet Dental Materials Co Ltd · Indian Institute of Technology Delhi · Institute Physical and Chemistry Materials De Strasbourg · Interface Biologics Inc · Louisiana State University |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Dental Implants and Abutments Pipeline Market by Segments
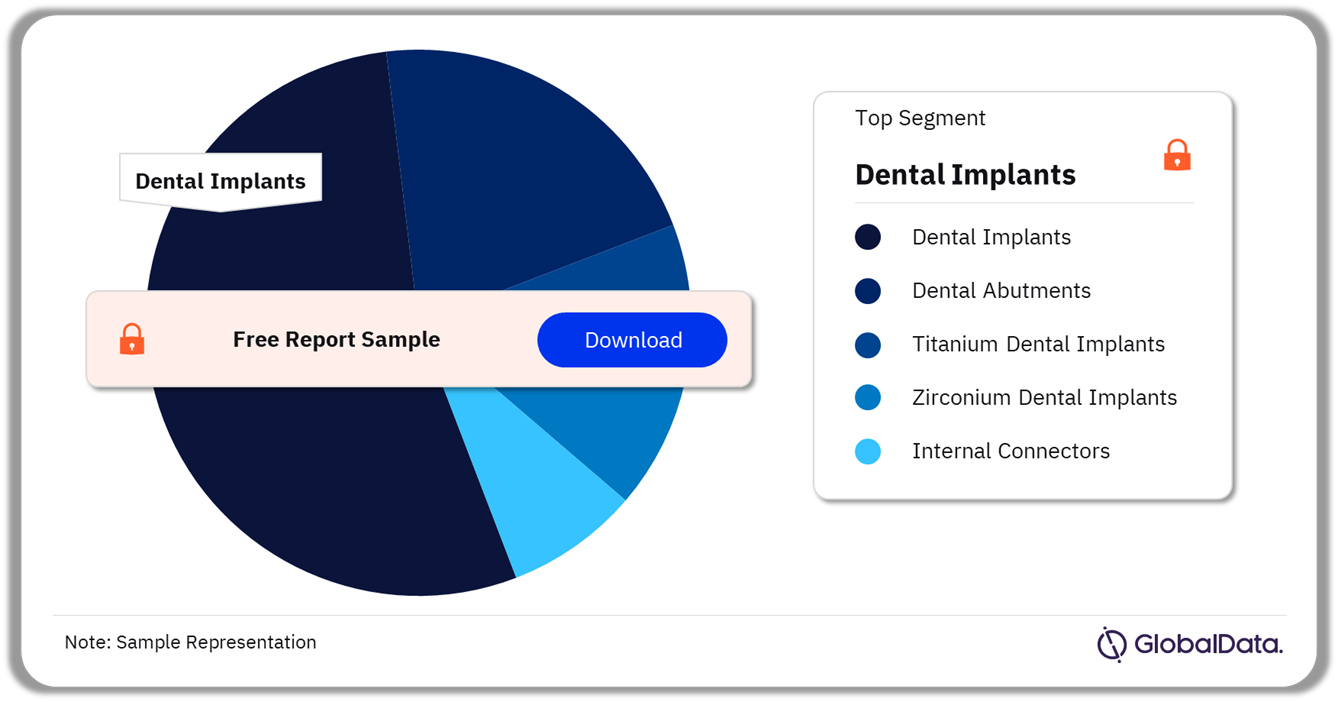
The key segments in the dental implants and abutments pipeline market are dental implants, dental abutments, titanium dental implants, zirconium dental implants, and internal connectors. As of October 2023, dental implants have the most pipeline products.
Dental Implants and Abutments Pipeline Market Analysis by Segments, 2023 (%)
Buy Full Report for More Segment Insights into the Dental Implants and Abutments Pipeline Market
Dental Implants and Abutments Pipeline Market Segmentation by Territories
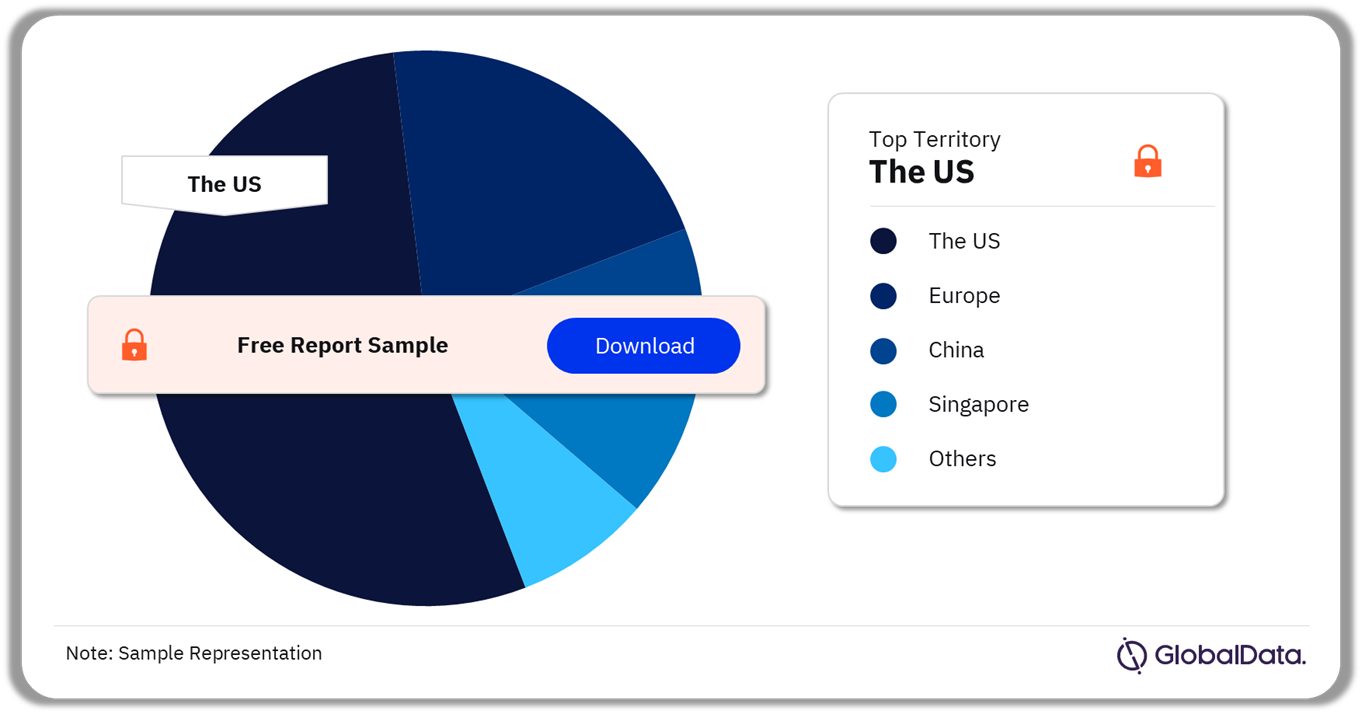
Some of the key territories in the dental implants and abutments market are the US, Europe, China, Singapore, Canada, Argentina, and Australia. As of October 2023, the US has the highest number of pipeline products.
Dental Implants and Abutments Pipeline Market Analysis by Territories, 2023 (%)
Buy Full Report for More Territory Insights into the Dental Implants and Abutments Pipeline Market
Dental Implants and Abutments Pipeline Market Segmentation by Regulatory Paths
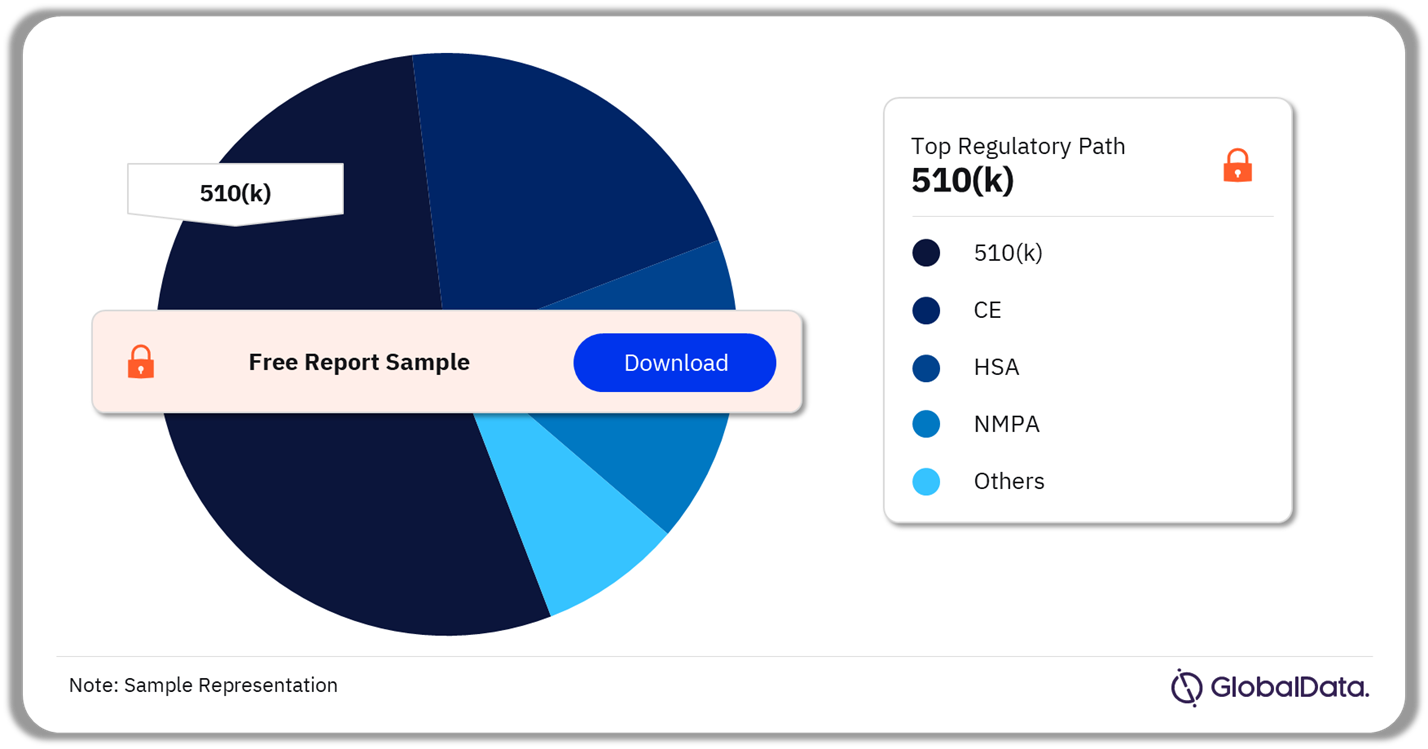
Some of the key regulatory paths in the dental implants and abutments pipeline market are 510(k), CE, HSA, NMPA, MDL, Ninsho, and BOPA. As of October 2023, 510(k) is the most followed pathway for pipeline products in the market.
Dental Implants and Abutments Pipeline Market Analysis by Regulatory Paths, 2023 (%)
Buy Full Report for More Regulatory Path Insights into the Dental Implants and Abutments Pipeline Market
Dental Implants and Abutments Pipeline Market – Competitive Landscape
Some of the key companies in the dental implants and abutments pipeline market are AddBio AB, BiteOn NV, Boston University, Coland Holdings Limited, Face Your Face HandelsGesmbH, Hefei Boyamet Dental Materials Co Ltd, Indian Institute of Technology Delhi, Institute Physical and Chemistry Materials De Strasbourg, Interface Biologics Inc, and Louisiana State University.
AddBio AB: It is a medical device company that develops solutions for weak bone problems in the implant industry. AddBio’s product finds application in dental implants which increases the stability and reduces the risk of complications and in trauma implants without causing removal problems from bone in-growth. The company operates through dental and orthopedic implant companies for the co-development of its technology. AddBio is headquartered in Linkoping, Sweden.
BiteOn NV: It is a medical equipment company that is engaged in the distribution and manufacture of dental devices and offers other related services. BiteOn is headquartered in Amsterdam, the Netherlands.
Dental Implants and Abutments Pipeline Market Analysis by Companies, 2023
Buy Full Report for More Company Insights into the Dental Implants and Abutments Pipeline Market
Segments Covered in the Report
Dental Implants and Abutments Pipeline Market Segments Outlook
- Dental Implants
- Dental Abutments
- Titanium Dental Implants
- Zirconium Dental Implants
- Internal Connectors
Dental Implants and Abutments Pipeline Market Territories Outlook
- The US
- Europe
- China
- Singapore
- Canada
- Argentina
- Australia
Dental Implants and Abutments Pipeline Market Regulatory Paths Outlook
- 510(k)
- CE
- HAS
- NMPA
- MDL
- Ninsho
- BOPA
Scope
This report provides:
- Extensive coverage of the Dental Implants and Abutments under development.
- Review details of major pipeline products which include product description, licensing and collaboration details and other developmental activities.
- Reviews of major players involved in the pipeline product development and lists all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from early development to approved/issued stage.
- Provides key clinical trial data related to ongoing clinical trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with potentially strong product portfolio and create effective counter strategies to gain a competitive advantage.
- Identify and understand important and diverse types of Dental Implants and Abutments under development.
- Develop market-entry and market-expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date.
BiteOn NV
Boston University
Coland Holdings Limited
Face Your Face HandelsGesmbH
Hefei Boyamet Dental Materials Co Ltd
Indian Institute of Technology Delhi
Institute Physical And Chemistry Materials De Strasbourg
Interface Biologics Inc
Louisiana State University
Medtronic Plc
MicroPort Scientific Corp
Nano Interface Technology LLC (Inactive)
Nanoker Research SL
National Dental Centre Singapore
Natural Dental Implants AG
Neodent SA
Nobel Biocare Services AG
Odontis Ltd (Inactive)
Osteopore International Pte Ltd
Pharmaco-Kinesis Corp
Preferred Dental Implant Corp
Rutgers The State University of New Jersey
Silver Bullet Therapeutics, Inc.
Sinclair Pharma Ltd
SINTX Technologies Inc
Straumann Holding AG
Taragenyx Ltd (Inactive)
Tissue Regeneration Systems, Inc.
Universitas Padjadjaran
University of Connecticut
University of Melbourne
University of Pennsylvania
University of Pittsburgh
University of Utah
Zeev Implants Ltd
ZimVie Inc
Table of Contents
Table
Figures
Frequently asked questions
-
Which is the leading segment in the dental implants and abutments pipeline market?
Dental implants is the leading segment in the dental implants and abutments pipeline market as of October 2023.
-
Which territory has the highest number of pipeline products in the dental implants and abutments pipeline market?
As of October 2023, the US has the highest number of products in the dental implants and abutments pipeline market.
-
Which is the most followed regulatory pathway in the dental implants and abutments pipeline market?
As of October 2023, 510(k) is the most followed pathway for pipeline products in the dental implants and abutments market.
-
Who are the major companies operating in the dental implants and abutments pipeline market?
Some of the key companies in the dental implants and abutments pipeline market are AddBio AB, BiteOn NV, Boston University, Coland Holdings Limited, Face Your Face HandelsGesmbH, Hefei Boyamet Dental Materials Co Ltd, Indian Institute of Technology Delhi, Institute Physical and Chemistry Materials De Strasbourg, Interface Biologics Inc, and Louisiana State University.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Dental Implants and Abutments reports