Diabetic Retinopathy Drugs in Development by Stages, Target, MoA, RoA, Molecule Type and Key Players, 2022 Update
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Diabetic Retinopathy Drugs Market Report Overview
Diabetic retinopathy is a complication of diabetes that affects the eyes. The symptoms may include spots or dark strings floating in vision (floaters), blurred vision, fluctuating vision, vision loss, and difficulty with color perception. The Diabetic Retinopathy pipeline drugs market research report provides comprehensive information on the therapeutics under development for Diabetic Retinopathy (Metabolic Disorders), complete with analysis by stage of development, drug target, mechanism of action (MoA), route of administration (RoA), and molecule type.
The report also covers the descriptive pharmacological action of the therapeutics, its complete research and development history, and the latest news and press releases. Additionally, the report reviews key players involved in therapeutic development for Diabetic Retinopathy and features dormant and discontinued projects.
| Key Targets | Vascular Endothelial Growth Factor A, Vascular Endothelial Growth Factor), Placenta Growth Factor, Melanocyte Stimulating Hormone Receptor, Angiopoietin 1 Receptor, Integrin Alpha V, Integrin Beta 3, Melanocortin Receptor 5, Semaphorin 3A, and ADP Ribosylation Factor 6 |
| Key Mechanisms of Action | Vascular Endothelial Growth Factor A Inhibitor, Vascular Endothelial Growth Factor Inhibitor, Placenta Growth Factor Inhibitor, Melanocyte Stimulating Hormone Receptor Agonist, Integrin Alpha V Antagonist, Integrin Beta 3 Antagonist, Melanocortin Receptor 5 Agonist, Semaphorin 3A Inhibitor, ADP Ribosylation Factor 6 Inhibitor, and Angiopoietin 1 Receptor Agonist |
| Key Routes of Administration | Intravitreal, Oral, Ophthalmic, Topical, Intravenous, Intraocular, Parenteral, Subcutaneous, Subconjunctival, and Buccal |
| Key Molecule Types | Small Molecule, Monoclonal Antibody, Fusion Protein, Synthetic Peptide, Biologic, Cell Therapy, Gene Therapy, Antisense RNAi Oligonucleotide, Recombinant Protein, and Aptamer |
| Leading Companies | F. Hoffmann-La Roche Ltd, Palatin Technologies Inc, Boehringer Ingelheim International GmbH, PharmAbcine Inc, Regeneron Pharmaceuticals Inc, A6 Pharmaceuticals LLC, Angios GmbH, Ascentage Pharma Group International, Bayer AG, and Charlesson LLC |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
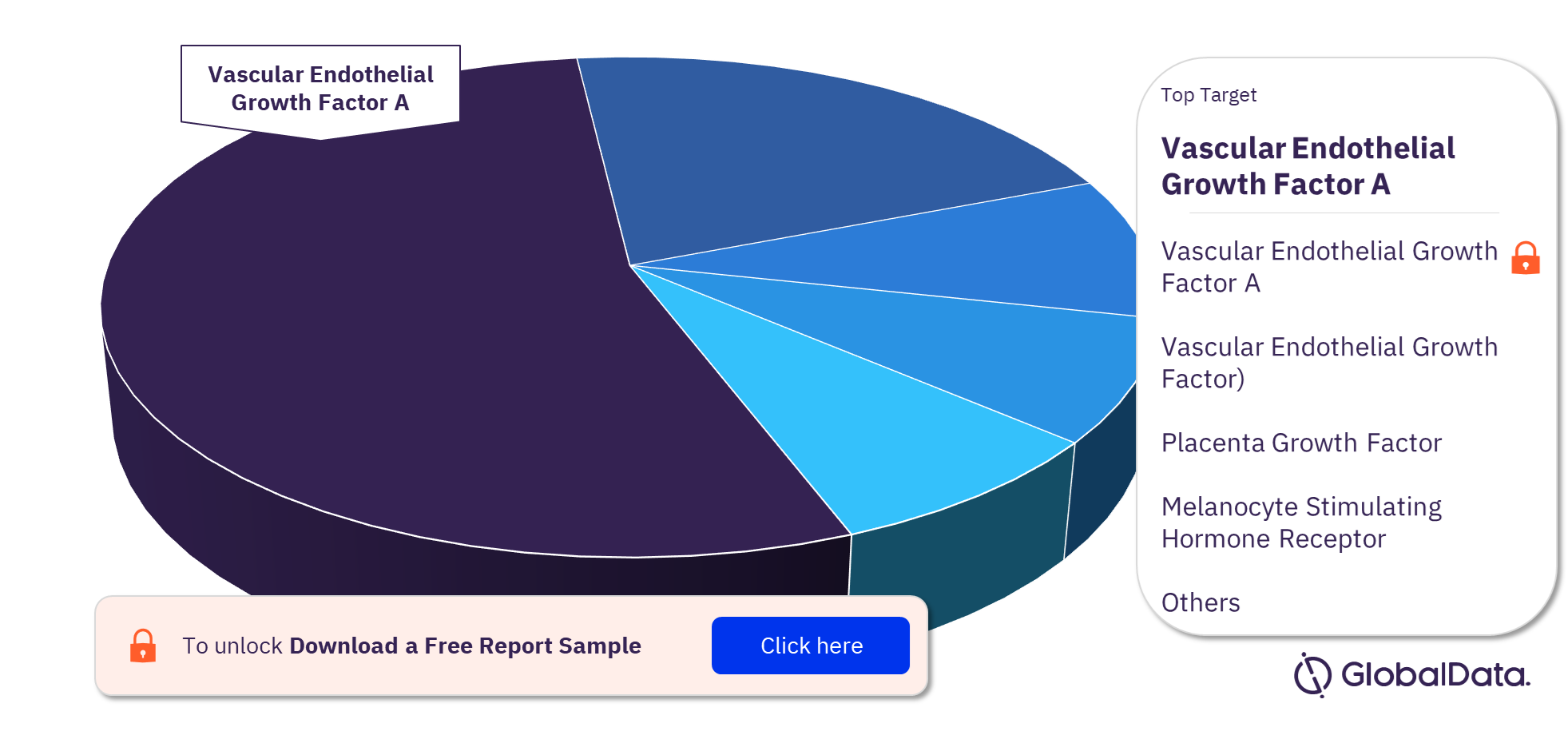
Diabetic Retinopathy Pipeline Drugs Market Segmentation by Targets
Some of the key targets of the Diabetic Retinopathy pipeline drugs market are Vascular Endothelial Growth Factor A, Vascular Endothelial Growth Factor), Placenta Growth Factor, Melanocyte Stimulating Hormone Receptor, Angiopoietin 1 Receptor, Integrin Alpha V, Integrin Beta 3, Melanocortin Receptor 5, Semaphorin 3A, and ADP Ribosylation Factor 6. In 2022, Vascular Endothelial Growth Factor A emerged as the most adopted target in the diabetic retinopathy pipeline drugs market.
Diabetic Retinopathy Pipeline Drugs Market Analysis by Targets, 2022 (%)
For more Diabetic Retinopathy pipeline drugs market target insights, download a free report sample
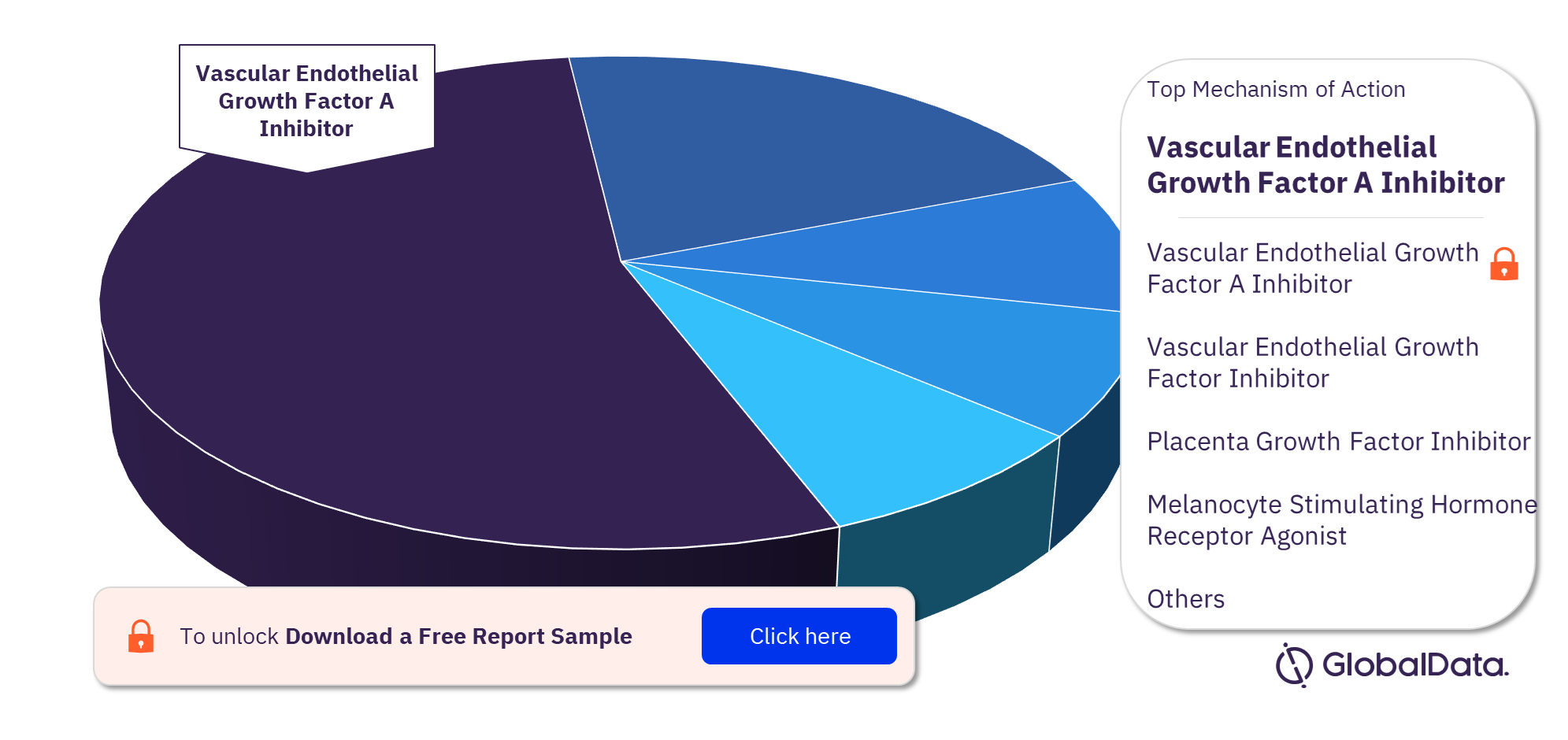
Diabetic Retinopathy Pipeline Drugs Market Segmentation by Mechanisms of Action
Some of the key mechanisms of action of the Diabetic Retinopathy pipeline drugs market are Vascular Endothelial Growth Factor A Inhibitor, Vascular Endothelial Growth Factor Inhibitor, Placenta Growth Factor Inhibitor, Melanocyte Stimulating Hormone Receptor Agonist, Integrin Alpha V Antagonist, Integrin Beta 3 Antagonist, Melanocortin Receptor 5 Agonist, Semaphorin 3A Inhibitor, ADP Ribosylation Factor 6 Inhibitor, and Angiopoietin 1 Receptor Agonist. In 2022, Vascular Endothelial Growth Factor A Inhibitor is the most adopted mechanism of action in the diabetic retinopathy pipeline drugs market.
Diabetic Retinopathy Pipeline Drugs Market Analysis by Mechanisms of Action, 2022 (%)
For more mechanism of action insights into the Diabetic Retinopathy pipeline drugs market, download a free report sample
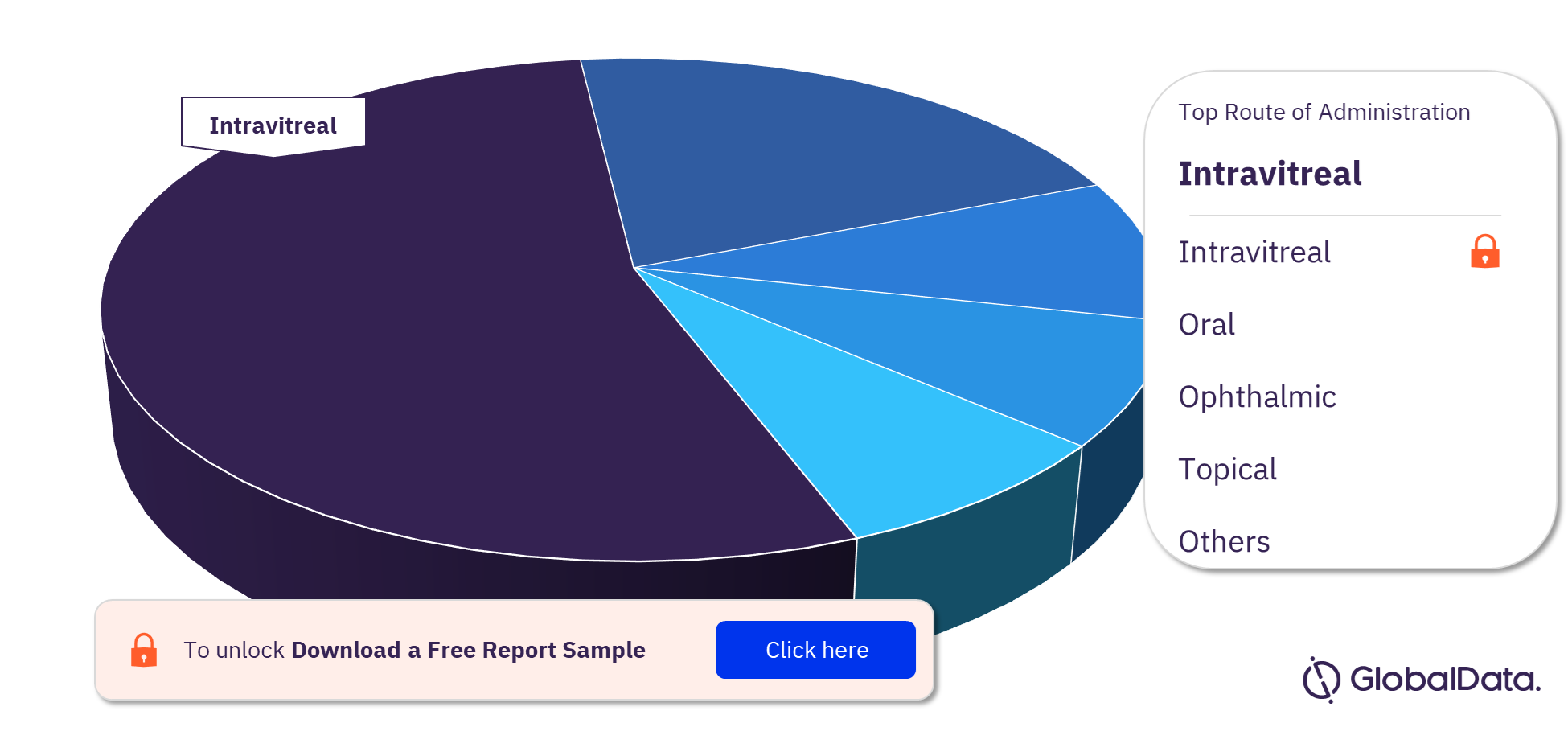
Diabetic Retinopathy Pipeline Drugs Market Segmentation by Routes of Administration
Some of the key routes of administration in the Diabetic Retinopathy pipeline drugs market are intravitreal, oral, ophthalmic, topical, intravenous, intraocular, parenteral, subcutaneous, subconjunctival, and buccal. In 2022, the most prominent route of administration for the diabetic retinopathy pipeline drugs market is intravitreal RoA.
Diabetic Retinopathy Pipeline Drugs Market Analysis by Routes of Administration, 2022 (%)
For more routes of administration insights into the Diabetic Retinopathy pipeline drugs market, download a free report sample
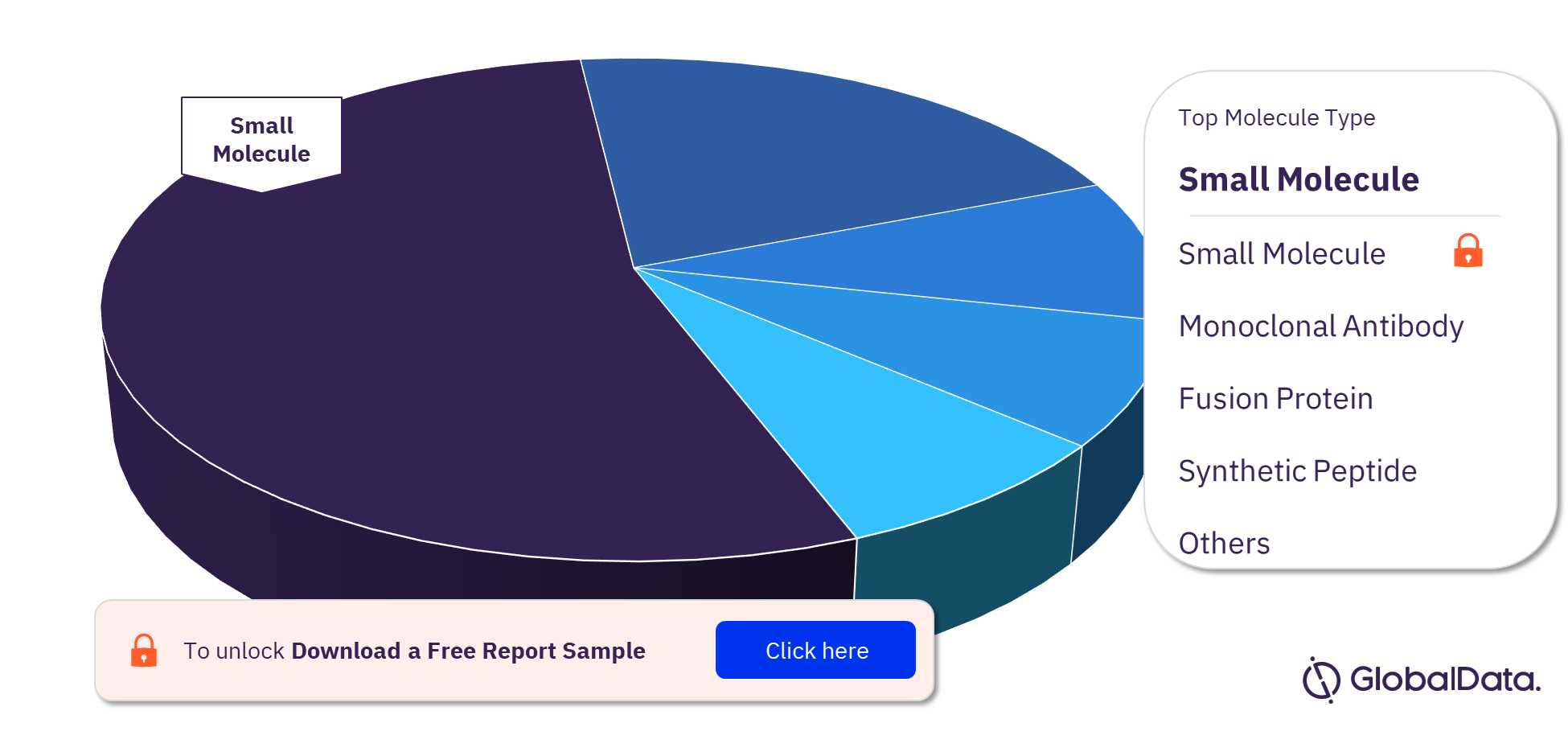
Diabetic Retinopathy Pipeline Drugs Market Segmentation by Molecule Types
The molecule types in the Diabetic Retinopathy pipeline drugs market are small molecule, monoclonal antibody, fusion protein, synthetic peptide, biologic, cell therapy, gene therapy, antisense RNAi oligonucleotide, recombinant protein, and aptamer. In 2022, the majority of the molecule types in the market are small molecules.
Diabetic Retinopathy Pipeline Drugs Market Analysis by Molecule Types, 2022 (%)
For more molecule type insights into the Diabetic Retinopathy pipeline drugs market, download a free report sample
Diabetic Retinopathy Pipeline Drugs Market - Competitive Landscape
Some of the leading companies in the Diabetic Retinopathy pipeline drugs market are F. Hoffmann-La Roche Ltd, Palatin Technologies Inc, Boehringer Ingelheim International GmbH, PharmAbcine Inc, Regeneron Pharmaceuticals Inc, A6 Pharmaceuticals LLC, Angios GmbH, Ascentage Pharma Group International, Bayer AG, and Charlesson LLC. In 2022, F. Hoffmann-La Roche Ltd emerged as the leading company with the highest number of pipeline drugs.
Diabetic Retinopathy Pipeline Drugs Market Analysis by Companies, 2022 (%)
To know more about the companies in the Diabetic Retinopathy pipeline drugs market, download a free report sample
Scope
This report provides:
- A snapshot of the global therapeutic landscape of Diabetic Retinopathy (Metabolic Disorders).
- Reviews of pipeline therapeutics for Diabetic Retinopathy (Metabolic Disorders) by companies and universities/research institutes based on information derived from company and industry-specific sources.
- Pipeline products based on several stages of development ranging from pre-registration to discovery and undisclosed stages.
- Descriptive drug profiles for the pipeline products which comprise product description, descriptive licensing and collaboration details, R&D brief, MoA & other developmental activities.
- Reviews of key companies involved in Diabetic Retinopathy (Metabolic Disorders) therapeutics and enlists all their major and minor projects.
- Evaluation of Diabetic Retinopathy (Metabolic Disorders) therapeutics based on mechanism of action (MoA), drug target, route of administration (RoA), and molecule type.
- All the dormant and discontinued pipeline projects.
- Reviews of the latest news related to pipeline therapeutics for Diabetic Retinopathy (Metabolic Disorders).
Reasons to Buy
- Procure strategically important competitor information, analysis, and insights to formulate effective R&D strategies.
- Recognize emerging players with potentially strong product portfolio and create effective counterstrategies to gain competitive advantage.
- Find and recognize significant and varied types of therapeutics under development for Diabetic Retinopathy (Metabolic Disorders).
- Classify potential new clients or partners in the target demographic.
- Develop tactical initiatives by understanding the focus areas of leading companies.
- Plan mergers and acquisitions meritoriously by identifying key players and their most promising pipeline therapeutics.
- Formulate corrective measures for pipeline projects by understanding Diabetic Retinopathy (Metabolic Disorders) pipeline depth and focus of Indication therapeutics.
- Develop and design in-licensing and out-licensing strategies by identifying prospective partners with the most attractive projects to enhance and expand business potential and scope.
- Adjust the therapeutic portfolio by recognizing discontinued projects and understand from the know-how what drove them from the pipeline.
AbbVie Inc
Aerie Pharmaceuticals Inc
Ailon Co Ltd
Amytrx Therapeutics Inc
Angios GmbH
AntlerA Therapeutics Inc
Apexian Pharmaceuticals Inc
Applied Therapeutics Inc
AptaBio Therapeutics Inc
Aptamer Sciences Inc
Araim Pharmaceuticals Inc
Arctic Vision Shanghai Biotechnology Co Ltd
Ascentage Pharma Group International
Athenex Inc
Aviceda Therapeutics Inc
Avirmax Inc
Bayer AG
BetaStem Therapeutics Inc
Bio-Thera Solutions Ltd
Boehringer Ingelheim International GmbH
Bonac Corp
Caregen Co Ltd
CCRP Therapeutics GmbH
Cell Care Therapeutics Inc
Celros Biotech Co Ltd
Charlesson LLC
Clayton Biotechnologies Inc
Coherus BioSciences Inc
Connexin Therapeutics Inc
Curative Biotechnology Inc
Eluminex Biosciences Ltd
Ennovabio
Epigen Biosciences Inc
Everglades Biopharma LLC
Excitant Therapeutics LLC
EyeGene Inc
EyePoint Pharmaceuticals Inc
F. Hoffmann-La Roche Ltd
Foresee Pharmaceuticals Co Ltd
Frontbio Co Ltd
Generium
Glycadia Inc
Grupo Ferrer Internacional SA
Guangzhou Magpie Pharmaceutical Co Ltd
HanAll Biopharma Co Ltd
ImmunAbs Inc
Inflammx Therapeutics Inc
iRegene Therapeutics Co Ltd
iVeena Delivery Systems Inc
jCyte Inc
Kalos Therapeutics Inc
Kanaph Therapeutics Inc
Kato Pharmaceuticals Inc
Kiora Pharmaceuticals Inc
Kodiak Sciences Inc
Kubota Vision Inc
Luye Pharma Group Ltd
Mabion SA
MD Healthcare Inc
MingSight Pharmaceuticals Inc
Mirae Cell Bio Co Ltd
NanoPharmaceuticals LLC
NB Health Laboratory Co Ltd
NeuMedics Inc
Neurodegeneration Therapeutics Inc
NGM Biopharmaceuticals Inc
Novago Therapeutics AG
Novartis AG
Novelty Nobility Inc
Noveome Biotherapeutics Inc
OccuRx Pty Ltd
Ocugen Inc
Ocular Therapeutix Inc
OcuNexus Therapeutics Inc
OcuTerra Therapeutics Inc
OliPass Corporation
OM Pharma Ltd
Opera Therapeutics
Oxurion NV
Palatin Technologies Inc
Phanes Therapeutics Inc
PharmAbcine Inc
Pinotbio Inc
Praetego Inc
Profarma
Ranger Biotechnologies AS
Regenerate Therapeutics Inc
Regeneron Pharmaceuticals Inc
RemeGen Co Ltd
Retinset SL
Retrotope Inc
Reven Holdings Inc
Ribomic Inc
Sciwind Biosciences Co Ltd
Serodus ASA
Shanghai Henlius Biotech Inc
Shenzhen Evergreen Therapeutics Co Ltd
Shilpa Medicare Ltd
SIFI SpA
Singh Biotechnology LLC
SiNOPSEE Therapeutics Pte Ltd
Skye Bioscience Inc
Skyran Biologics Inc
SmartinBio
SunBio Inc
Sylentis SAU
Talem Therapeutics Inc
Teraclon IDF SL
ToolGen Inc
Topadur Pharma AG
Translatum Medicus Inc
Unity Biotechnology Inc
USA Elixiria Biotech Inc
Valitor Inc
Valo Health LLC
Vascugen Inc
VESSL Therapeutics Ltd
Vitreo Pharma Inc
Wntgen LLC
Xbrane Biopharma AB
Xequel Bio Inc
YD Life Science Co
Zih Yuan Tang Biotechnology Co Ltd
Table of Contents
Table
Figures
Frequently asked questions
-
What are the key targets of the Diabetic Retinopathy pipeline drugs market?
Some of the key targets of the Diabetic Retinopathy pipeline drugs market are Vascular Endothelial Growth Factor A, Vascular Endothelial Growth Factor), Placenta Growth Factor, Melanocyte Stimulating Hormone Receptor, Angiopoietin 1 Receptor, Integrin Alpha V, Integrin Beta 3, Melanocortin Receptor 5, Semaphorin 3A, and ADP Ribosylation Factor 6.
-
What are the key mechanisms of action of the Diabetic Retinopathy pipeline drugs market?
Some of the key mechanisms of action of the Diabetic Retinopathy pipeline drugs market are Vascular Endothelial Growth Factor A Inhibitor, Vascular Endothelial Growth Factor Inhibitor, Placenta Growth Factor Inhibitor, Melanocyte Stimulating Hormone Receptor Agonist, Integrin Alpha V Antagonist, Integrin Beta 3 Antagonist, Melanocortin Receptor 5 Agonist, Semaphorin 3A Inhibitor, ADP Ribosylation Factor 6 Inhibitor, and Angiopoietin 1 Receptor Agonist.
-
What are the key routes of administration in the Diabetic Retinopathy pipeline drugs market?
Some of the key routes of administration in the Diabetic Retinopathy pipeline drugs market are intravitreal, oral, ophthalmic, topical, intravenous, intraocular, parenteral, subcutaneous, subconjunctival, and buccal.
-
What are the key molecule types in the Diabetic Retinopathy pipeline drugs market?
The molecule types in the Diabetic Retinopathy pipeline drugs market are small molecule, monoclonal antibody, fusion protein, synthetic peptide, biologic, cell therapy, gene therapy, antisense RNAi oligonucleotide, recombinant protein, and aptamer.
-
Which are the leading companies in the Diabetic Retinopathy pipeline drugs market?
Some of the leading companies in the Diabetic Retinopathy pipeline drugs market are F. Hoffmann-La Roche Ltd, Palatin Technologies Inc, Boehringer Ingelheim International GmbH, PharmAbcine Inc, Regeneron Pharmaceuticals Inc, A6 Pharmaceuticals LLC, Angios GmbH, Ascentage Pharma Group International, Bayer AG, and Charlesson LLC.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Metabolic Disorders reports