Drugs of Abuse Pipeline Report including Stages of Development, Segments, Region and Countries, Regulatory Path and Key Companies, 2022 Update
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Drugs of abuse tests are used for the detection of illegal and/or prescribed substances in the patent samples including urine, blood, saliva, or sweat. The drugs of abuse pipeline market research report provide comprehensive information about the drugs of abuse pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials which are in progress. Moreover, the report provides information about various pipeline products and their estimated approval dates.
Drugs of Abuse Pipeline Products Market Segmentation by Territories
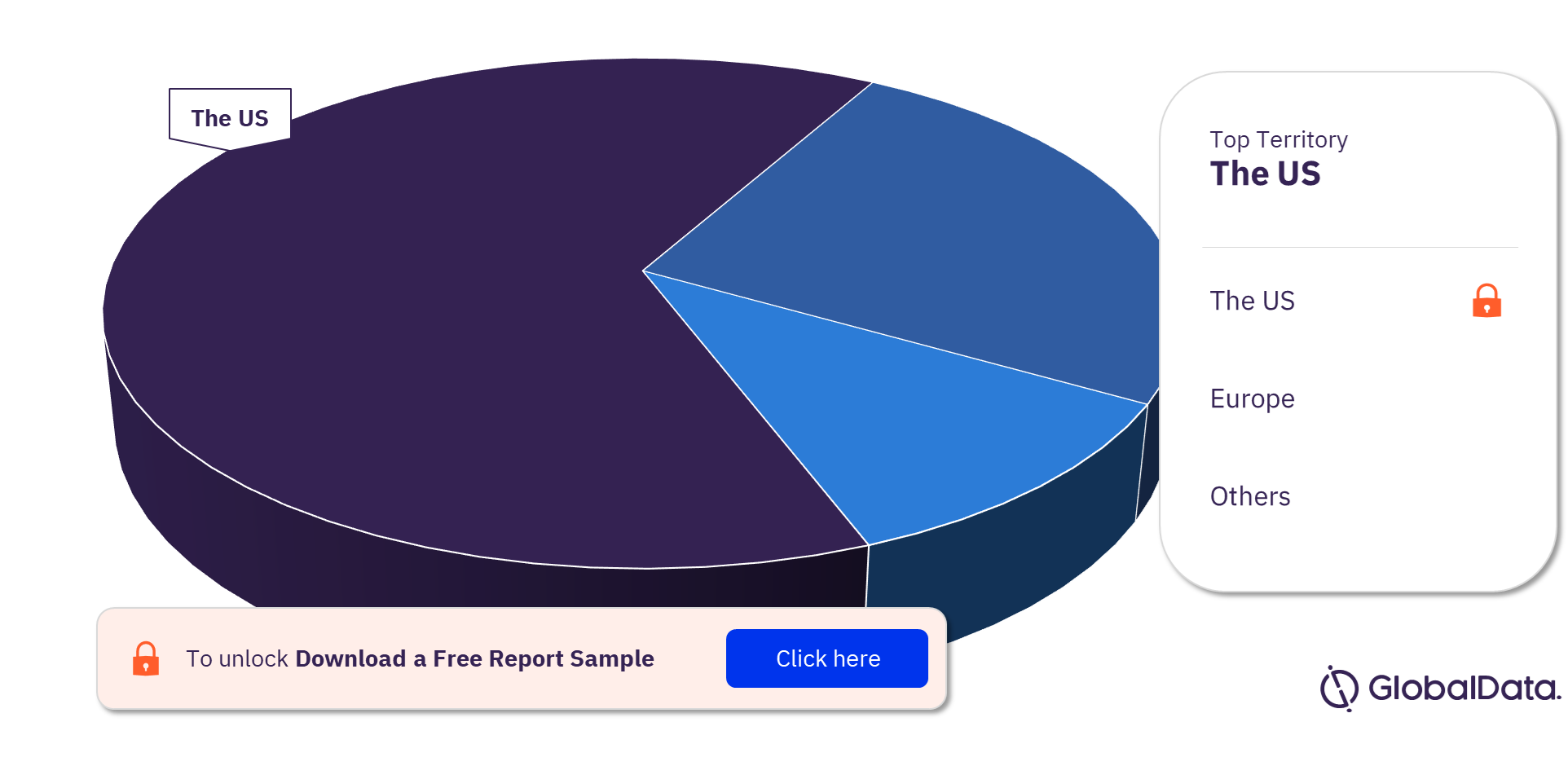
The key territories with products in the pipeline are the US and Europe, among others. The US is the leading territory in the drugs of abuse pipeline products market.
Drugs of Abuse Pipeline Products Market Analysis by Territories, 2022 (%)
For more territory insights into the drugs of abuse pipeline products market, download a free report sample
Drugs of Abuse Pipeline Products Market Segmentation by Regulatory Paths
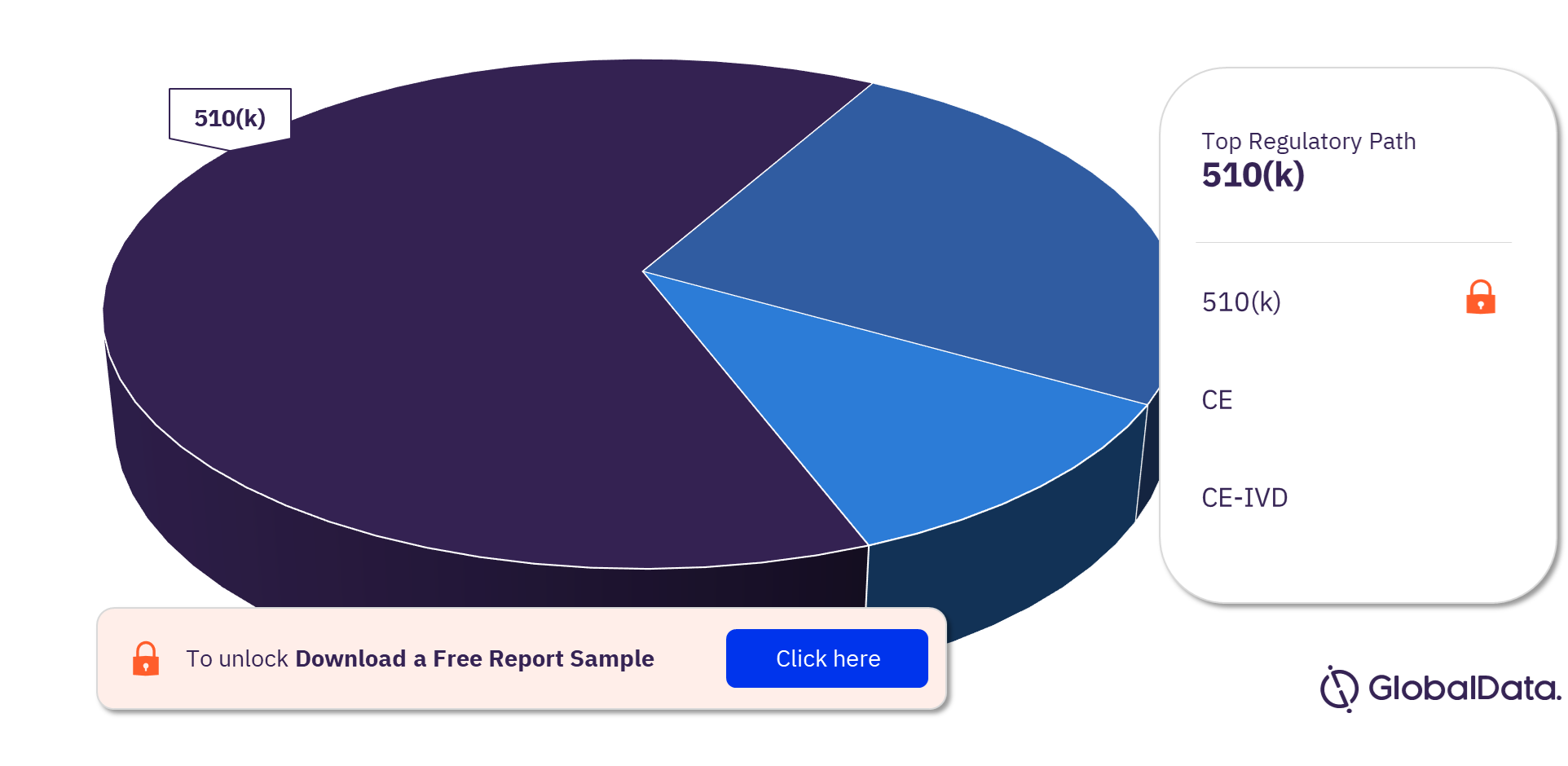
The drugs of abuse pipeline report provide detailed insights into the pipeline products by a regulatory path including 510(k), CE, and CE-IVD. Most of the products follow the 510(k) pathway to enter the market.
Drugs of Abuse Pipeline Products Market Analysis by Regulatory Paths, 2022 (%)
For more regulatory path insights into the drugs of abuse pipeline products market, download a free report sample
Drugs of Abuse Pipeline Products Market - Competitive Landscape
Some of the leading companies in the drugs of abuse pipeline products market are Accelerate Diagnostics Inc, Adaltis Srl, Banyan Biomarkers Inc, Behavioral Diagnostics Inc, BioMark Technologies Inc, Ceres Nanosciences Inc, genedrive plc, Horiba Ltd, OpalGenix Inc, Randox Laboratories Ltd, Roche Diagnostics International Ltd, and SDI Biomed Inc.
Accelerate Diagnostics Inc: Accelerate Diagnostics Inc (Accelerate) is an in vitro diagnostics company engaged in the development and commercialization of instruments for the identification and testing of antibiotic susceptibility to infectious pathogens. The company operates a manufacturing facility in Tucson, Arizona. It sells products directly and through a network of third-party distributors to hospital microbiology laboratories in domestic and foreign countries.
Adaltis Srl: Adaltis Srl (Adaltis), a subsidiary of BATM Advanced Communications Ltd, is an international IVD company that develops, manufactures, and markets in-vitro diagnostic systems and reagents. The company’s products include IVD instruments, IVD reagents, molecular diagnostic instruments, molecular diagnostic tests, accessories, consumables, and spare parts.
Banyan Biomarkers Inc: Banyan Biomarkers Inc (Banyan Biomarkers) is a medical technology company that commercializes vitro diagnostic tests for the detection of traumatic brain injury and neurological diseases. The company’s product pipeline includes biomarkers for depression, hypoxic ischemic encephalopathy, neuro ICU monitoring, sub-acute or chronic traumatic brain injury, and stroke.
Drugs of Abuse Pipeline Products Market Report Overview
| Key Territories | The US and Europe |
| Analysis Year | 2022 |
| Key Regulatory Paths | 510(k), CE, and CE-IVD |
| Leading Companies | Accelerate Diagnostics Inc, Adaltis Srl, Banyan Biomarkers Inc, Behavioral Diagnostics Inc, BioMark Technologies Inc, Ceres Nanosciences Inc, genedrive plc, Horiba Ltd, OpalGenix Inc, Randox Laboratories Ltd, Roche Diagnostics International Ltd, and SDI Biomed Inc |
Drugs of Abuse Pipeline Products Market Territories Outlook (2022)
- The US
- Europe
Drugs of Abuse Pipeline Products Market Regulatory Paths Outlook (2022)
- 510(k)
- CE
- CE-IVD
Scope
- Extensive coverage of the drugs of abuse under development
- The report reviews details of major pipeline products which includes, product description, licensing and collaboration details, and other developmental activities
- The report reviews the major players involved in the development of drugs of abuse and lists all their pipeline projects
- The coverage of pipeline products is based on various stages of development ranging from early development to the approved/issued stage
- The report provides key clinical trial data of ongoing trials specific to pipeline products
- Recent developments in the segment/industry
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies
- Identify emerging players with potentially strong product portfolios and create effective counter strategies to gain a competitive advantage
- Identify and understand important and diverse types of drugs of abuse under development
- Develop market-entry and market expansion strategies
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date
Adaltis Srl
Banyan Biomarkers Inc
Behavioral Diagnostics Inc
BioMark Technologies Inc
Ceres Nanosciences Inc
genedrive plc
Horiba Ltd
OpalGenix Inc
Randox Laboratories Ltd
Roche Diagnostics International Ltd
SDI Biomed, Inc.
SensoDXII LLC
Siemens Healthcare Diagnostics Inc
Siemens Healthineers AG
Thermo Fisher Scientific Inc
University of California San Francisco
ZORA Biosciences Oy
Table of Contents
Table
Figures
Frequently asked questions
-
Which are the key territories in the drugs of abuse pipeline products market?
The key territories in the drugs of abuse pipeline products market are the US and Europe, among others.
-
Which is the leading territory in the drugs of abuse pipeline products market?
The US is the leading territory in the drugs of abuse pipeline products market.
-
What are the key regulatory paths in the drugs of abuse pipeline products market?
The key regulatory paths in the drugs of abuse pipeline products market are 510(k), CE, and CE-IVD.
-
Which are the leading companies in the drugs of abuse pipeline products market?
Some of the leading companies in the drugs of abuse pipeline products market are Accelerate Diagnostics Inc, Adaltis Srl, Banyan Biomarkers Inc, Behavioral Diagnostics Inc, BioMark Technologies Inc, Ceres Nanosciences Inc, genedrive plc, Horiba Ltd, OpalGenix Inc, Randox Laboratories Ltd, Roche Diagnostics International Ltd, and SDI Biomed Inc.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more In Vitro Diagnostics reports