ECG Monitoring Equipment Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Electrocardiogram (ECG) Monitoring Equipment Pipeline Market Report Overview
Electrocardiogram (ECG) Monitoring Equipment records the electrocardiogram (ECG) and is used to evaluate 10 seconds (or a specific time interval) of electrical activity of the heart so a physician can determine the condition of a patient’s heart. The ECG Monitoring Equipment pipeline market research report provides comprehensive information about the ECG Monitoring Equipment pipeline products with comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
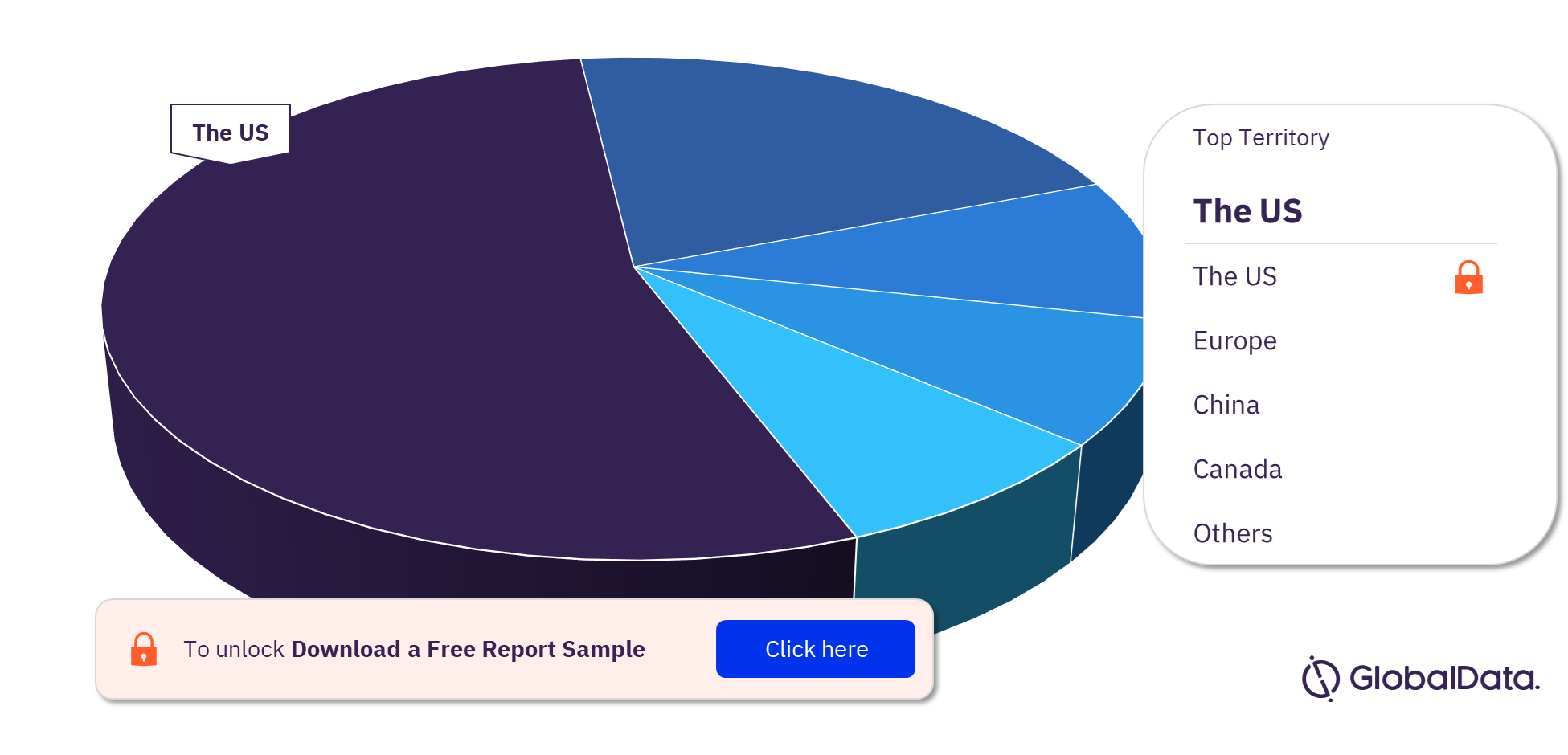
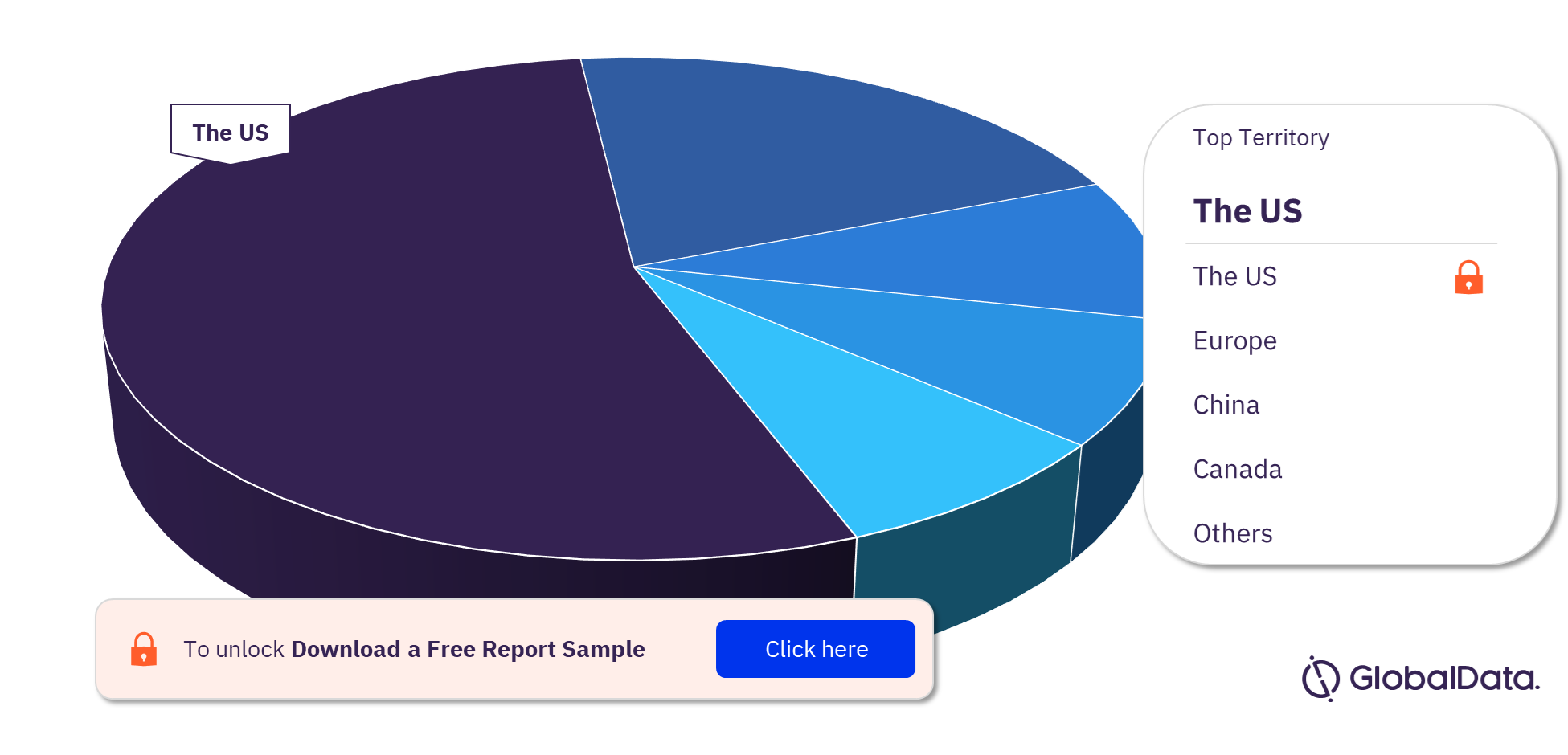
ECG Monitoring Equipment Pipeline Market Segmentation by Territories
Some of the key territories in the ECG Monitoring Equipment pipeline market are the US, Europe, China, Canada, India, and Japan. As of April 2023, the US has the highest number of pipeline products, followed by Europe and China.
ECG Monitoring Equipment Pipeline Market Analysis, by Territories
For more territory insights into the ECG Monitoring Equipment pipeline market, download a free report sample
ECG Monitoring Equipment Pipeline Market Segmentation by Regulatory Paths
Some of the key regulatory paths in the ECG Monitoring Equipment pipeline market are 510(k), CE, NMPA, ICAC, MDL, and Ninsho. As of April 2023, 510(k) is the most followed pathway for pipeline products.
ECG Monitoring Equipment Pipeline Market Analysis, by Regulatory Paths, 2023(%)
For more regulatory path insights into the ECG Monitoring Equipment pipeline market, download a free report sample
ECG Monitoring Equipment Pipeline Market – Competitive Landscape
Some of the key companies in the ECG Monitoring Equipment pipeline market are AccurKardia Inc, Advanced Medical Electronics Corp, Agatsa Software Pvt Ltd, AliveCor Inc, Appsens AS, Arizona State University, Auscultech Dx, Ben-Gurion University of the Negev, BioSense, and Biotricity Inc.
Advanced Medical Electronics Corp: Advanced Medical Electronics Corp (AME) is a medical device company that provide research and development services. The company‘s products include electrocardiograph and spirometer equipment. It also provides battery powered surgical headlamp, wrist-worn pulse oximeter, electroencephalograph, partial polysomnograph, diagnostic spirometer and computerized visual acuity testing. AME offers services such as systems design, embedded design, electronics and instrumentation design, wireless design, software development, prototype build, and system testing.
AliveCor Inc: AliveCor Inc (AliveCor) is a medical device and artificial intelligence company that develops and manufactures ECG hardware and software for mobile devices. The company provides Kardia, an AI enabled platform to help clinicians manage patients for the detection of atrial fibrillation and normal sinus rhythm in an ECG. It offers KardiaMobile and KardiaBand applications. AliveCor provides portable devices, early detections, immediate feedback and convenient short-term and long-term self-monitoring solutions.
Ben-Gurion University of the Negev: Ben-Gurion University of the Negev (Ben-Gurion University) is a public research university that offers educational and research programs and services. The university offers services such as undergraduate and graduate degrees, research in the field of green energy, alternative and renewable energy including renewable fuels and solar energy, environmental research, laboratories services, national and multi-disciplinary research services.
ECG Monitoring Equipment Pipeline Market Report Overview
| Key Territories | The US, Europe, China, Canada, India, and Japan |
| Key Regulatory Paths | 510(k), CE, NMPA, ICAC, MDL, and Ninsho |
| Leading Companies | AccurKardia Inc, Advanced Medical Electronics Corp, Agatsa Software Pvt Ltd, AliveCor Inc, Appsens AS, Arizona State University, Auscultech Dx, Ben-Gurion University of the Negev, BioSense, and Biotricity Inc |
Segments Covered in the Report
ECG Monitoring Equipment Pipeline Market Territories Outlook
- The United States
- Europe
- China
- Canada
- India
- Israel
- Japan
- Australia
- Argentina
- Chile
- New Zealand
- Peru
- Russia
- Singapore
- South Korea
- Taiwan
- The United Arab Emirates
- The United Kingdom
ECG Monitoring Equipment Pipeline Market Regulatory Paths Outlook
- 510(k)
- CE
- NMPA
- ICAC
- MDL
- Ninsho
- TGA
- UKCA
- MDITAC
- de novo
- HSA
- BOPA
Scope
- Extensive coverage of the ECG Monitoring Equipment under development.
- The report reviews details of major pipeline products which includes, product description, licensing and collaboration details and other developmental activities.
- The report reviews the major players involved in the development of ECG Monitoring Equipment and list all their pipeline projects.
- The coverage of pipeline products based on various stages of development ranging from Early Development to Approved / Issued stage.
- The report provides key clinical trial data of ongoing trials specific to pipeline products
- Recent developments in the segment / industry.
Reasons to Buy
The ECG Monitoring Equipment pipeline market report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with potentially strong product portfolio and create effective counter strategies to gain competitive advantage.
- Identify and understand important and diverse types of ECG Monitoring Equipment under development.
- Develop market-entry and market expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- In-depth analysis of the product’s current stage of development, territory and estimated launch date.
Advanced Medical Electronics Corp
Agatsa Software Pvt Ltd
AliveCor Inc
Appsens AS
Arizona State University
Auscultech Dx
Ben-Gurion University of the Negev
BioSense
Biotricity Inc
Boston Scientific Corp
Cambridge Heartwear Ltd
CardioComm Solutions Inc
Cirtec Medical Corp
Cognionics, Inc.
Coland Holdings Limited
Cybernet Medical
EasyG LLC
Emory University
Endotronix Inc
George Washington University
Global Instrumentation LLC
GrekTek LLC
HEARD Medical
Heart Health, Inc.
Heart Test Laboratories Inc
Heart Tronics, Inc. (Inactive)
HeartBeam Inc
HelpWear Inc
Huawei Technologies Co Ltd
IMEXCO General Ltd
iRhythm Technologies Inc
Johns Hopkins University
K2 Medical Ltd
LifeWatch Technologies, Ltd.
LivaNova PLC
Lundquist
Masimo Corp
Massachusetts Institute of Technology
MedBeat Sweden AB
Medical Care Technologies, Inc.
Medi-Lynx Cardiac Monitoring LLC
Medtronic Plc
Mezoo Co Ltd
MicroPort Scientific Corp
Minnesota Health Solutions Corporation
MiRTLE Medical LLC
MobilECG Laboratories Ltd.
NewCardio, Inc. (Inactive)
Nihon Kohden Corp
NimbleHeart Inc.
Novosense AB
Orbital Research Inc.
Peacs BV
Polytechnic University of Catalonia
Pravartan Technologies Pvt Ltd
Preventice Inc
Rapid Rhythm Ltd
Sensencall AS
Shimmer Research Ltd
Smart Solutions Technologies SL
Tempus Health Inc
Ten3t Healthcare Pvt Ltd
The Lundquist Institute
Toumaz Technology Limited
Trivitron Healthcare Pvt Ltd
University of Michigan
University of Michigan Pediatric Device Consortium
University of Tampere
University of Utah
Wellysis Corp
ZBeats Inc
Table of Contents
Table
Figures
Frequently asked questions
-
What is the major usage of ECG Monitoring Equipment?
Electrocardiogram (ECG) Monitoring Equipment records the electrocardiogram (ECG) and is used to evaluate 10 seconds (or a specific time interval) of electrical activity of the heart so a physician can determine the condition of a patient’s heart.
-
Which territory has the highest number of pipeline products in the ECG Monitoring Equipment market As of April 2023?
The US has the highest number of pipeline products in the ECG Monitoring Equipment market As of April 2023.
-
Which is the most followed regulatory pathway in the ECG Monitoring Equipment market As of April 2023?
As of April 2023, 510(k) is the most followed pathway in the ECG Monitoring Equipment pipeline market.
-
Which are the major players operating in the ECG Monitoring Equipment pipeline market?
Some of the major players operating in the ECG Monitoring Equipment pipeline market are AccurKardia Inc, Advanced Medical Electronics Corp, Agatsa Software Pvt Ltd, AliveCor Inc, Appsens AS, Arizona State University, Auscultech Dx, Ben-Gurion University of the Negev, BioSense, and Biotricity Inc.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more ECG Monitoring Equipment reports