Fetal Monitors Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Fetal Monitors Pipeline Market Overview
Fetal Monitors are devices used to monitor the fetal heart rate for indicators of stress, usually during labor and birth using ultrasound and electrocardiography. This is essentially a device for recording the uterine contractions during labor, and also the fetal heart rate, to monitor the progress of labor, and in particular the wellbeing of the fetus. This category includes antepartum and intrapartum monitors. This category includes the monitors only and the accessories associated with monitoring are not tracked under it.
The Fetal Monitors pipeline market research report provides comprehensive information about the pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials that are in progress.
| Key Segments | · Antepartum Monitors
· Intrapartum Monitors |
| Key Territories | · The US
· Australia · Europe · Saudi Arabia · India · United Kingdom |
| Key Regulatory Paths | · 510(k)
· ICAC · CE · TGA · UKCA · HDE Approvals |
| Leading Companies | · Applied Physics Systems Inc
· BardyDx Inc · BCB Informatica Y Control SL · Biorithm Pte Ltd · Biotricity Inc |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Fetal Monitors Pipeline Market Segmentation by Segments
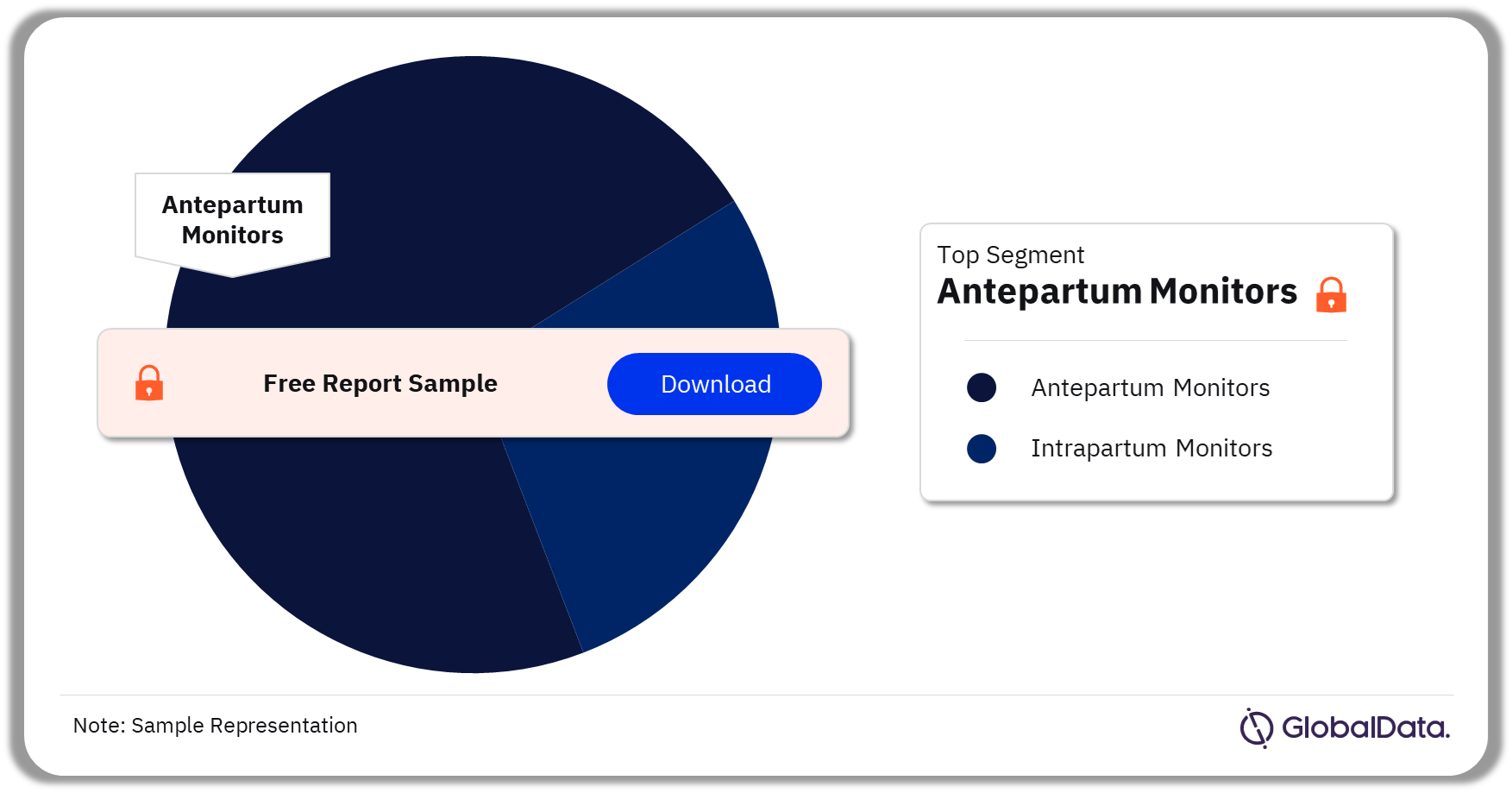
Some of the key segments in the Fetal Monitors market are antepartum monitors and intrapartum monitors. As of August 2023, antepartum monitors is the leading segment in the market.
Fetal Monitors Pipeline Market Analysis by Segments, 2023 (%)
Buy Full Report for More Segment Insights into The Fetal Monitors Pipeline Market, Download a Free Report Sample
Fetal Monitors Pipeline Market Segmentation by Territories
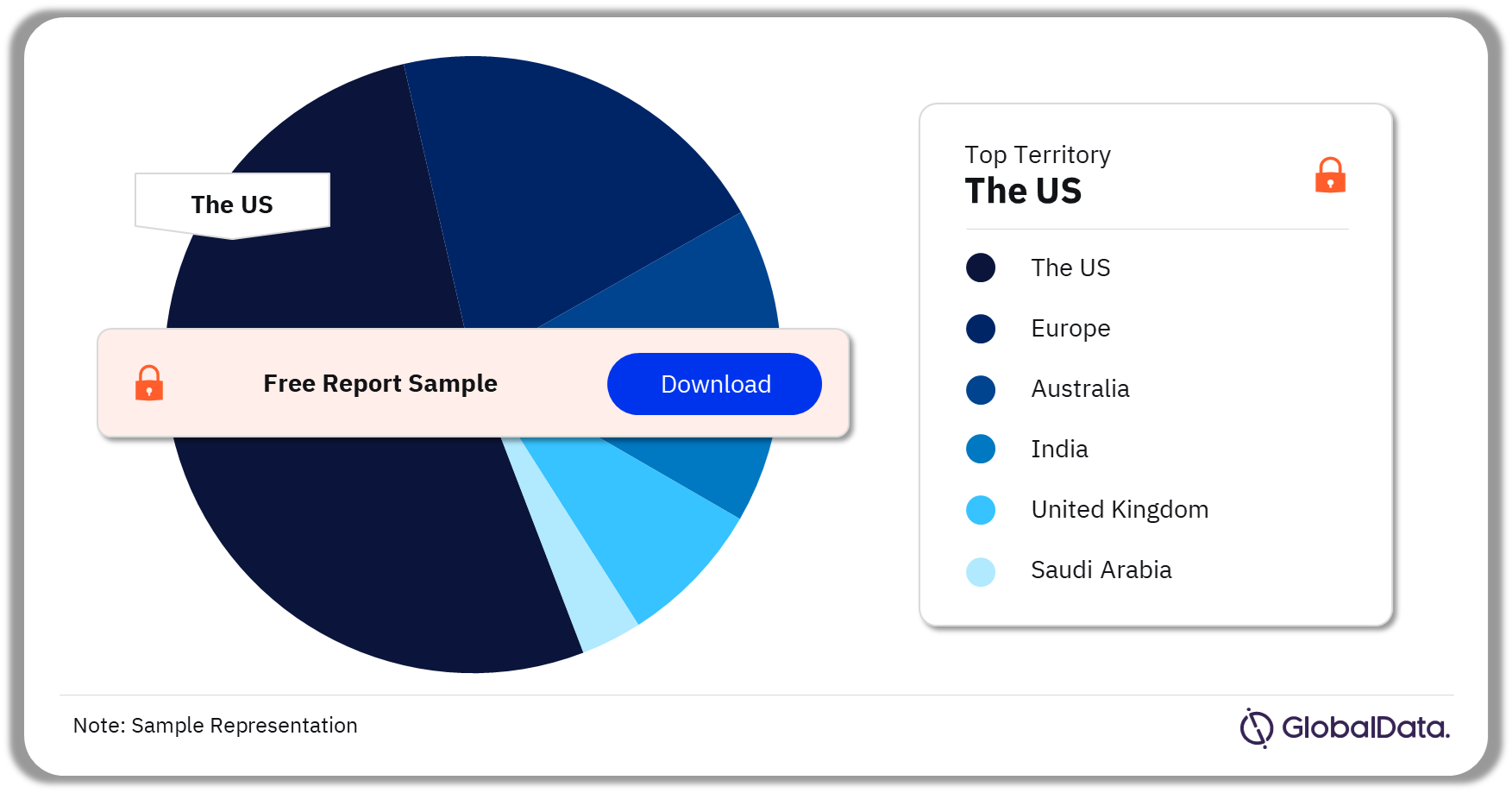
Some of the key territories in the Fetal Monitors market are the US, Australia, , Europe, India, United Kingdom, and Saudi Arabia. As of August 2023, the US accounts for the highest number of Fetal Monitors pipeline products.
Fetal Monitors Pipeline Market Analysis by Territories, 2023 (%)
Buy Full Report for More Territory Insights into The Fetal Monitors Pipeline Market, Download a Free Report Sample
Fetal Monitors Pipeline Market Segmentation by Regulatory Paths
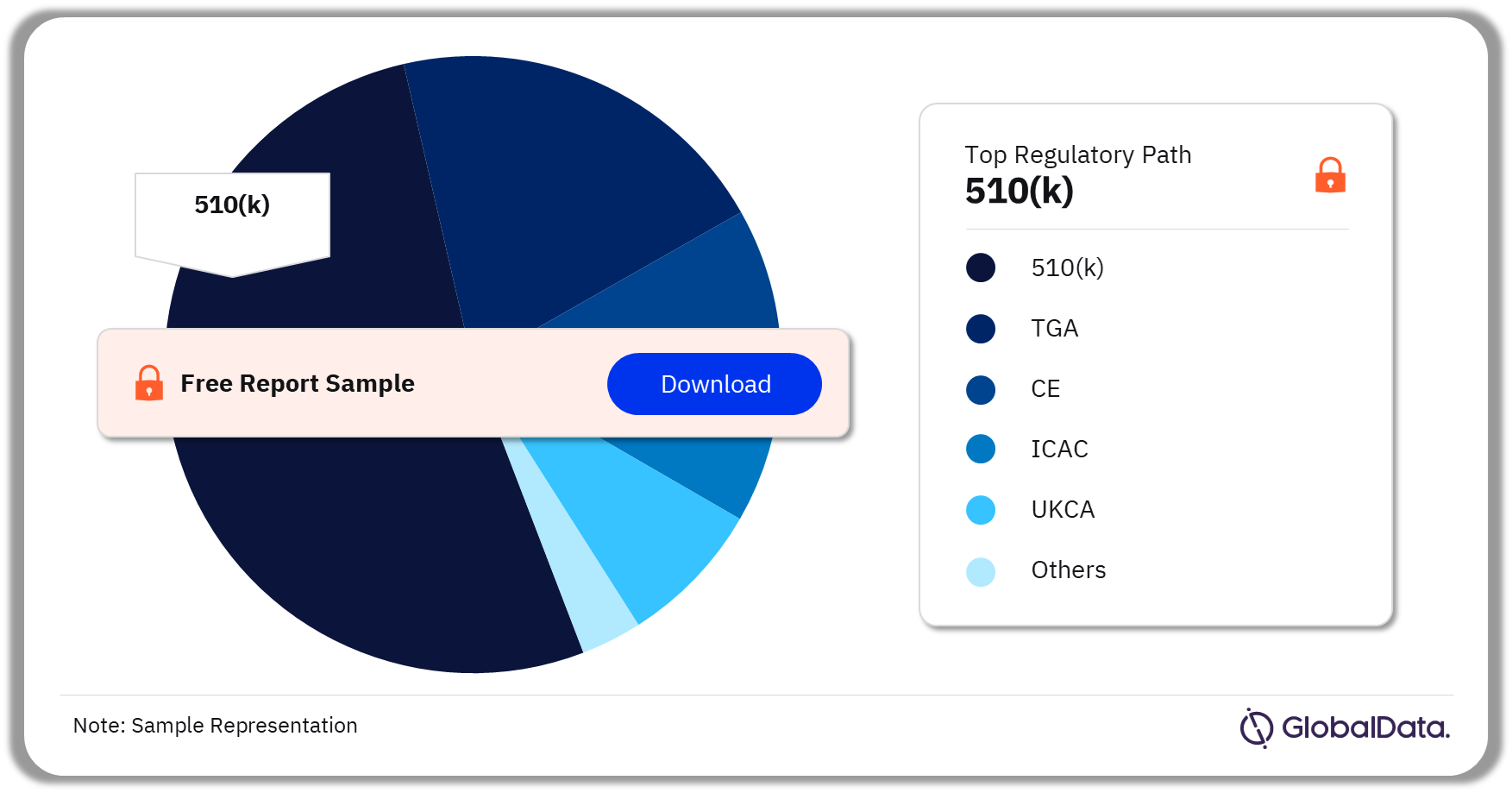
Some of the key regulatory paths in the Fetal Monitors pipeline market are 510(k), ICAC, CE, TGA, and UKCA among others. As of August 2023, 510(k) is the most followed pathway for pipeline products.
Fetal Monitors Pipeline Market Analysis by Regulatory Paths, 2023 (%)
Buy Full Report for More Regulatory Path Insights into The Fetal Monitors Pipeline Market, Download a Free Report Sample
Fetal Monitors Pipeline Market – Competitive Landscape
Some of the key companies in the Fetal Monitors pipeline market are Applied Physics Systems Inc., BardyDx Inc., BCB Informatica Y Control SL, Biorithm Pte Ltd, and Biotricity Inc. among others.
Biotricity Inc: Biotricity Inc (Biotricity) is a medical technology company. It offers biometric data monitoring solutions. It develops a Bioflux mobile cardiac telemetry solution that provides recurring reimbursements to doctors, hospitals, and independent diagnostic testing facilities. The revenue model that fits within the established insurance billing practices provides motion tracking to detect exercise, activity, and disorientation. In addition, Biotricity offers developing Biolife, a health and lifestyle solution for individuals. The company has a collaboration agreement with the university to determine the predictive value of electrocardiogram readings in preventative healthcare applications. Biotricity is headquartered in Toronto, Ontario, Canada.
Leading Fetal Monitors Companies, 2023
Buy Full Report for More Company Insights into The Fetal Monitors Pipeline Market, Download a Free Report Sample
Fetal Monitors Pipeline Market Segments
Fetal Monitors Pipeline Market Segments Outlook, (%, 2023)
- Antepartum monitors
- Intrapartum monitors
Fetal Monitors Pipeline Market Territories Outlook, (%, 2023)
- The US
- Australia
- Europe
- Saudi Arabia
- India
Fetal Monitors Pipeline Market Regulatory Paths Outlook, (%, 2023)
- ICAC
- 510(k)
- CE
- TGA
- UKCA
Scope
This report provides:
- Extensive coverage of the Fetal Monitors under development.
- Details of major pipeline products which include product description, licensing and collaboration details, and other developmental activities.
- Reviews of the major players involved in the development of Fetal Monitors and lists of all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from Early Development to Approved/Issued stage.
- Key clinical trial data of ongoing trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with potentially strong product portfolios and create effective counter strategies to gain a competitive advantage.
- Identify and understand important and diverse types of Fetal Monitors under development.
- Develop market-entry and market-expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date.
BardyDx Inc
BCB Informatica Y Control SL
Biorithm Pte Ltd
Biotricity Inc
Bloom Technologies Pvt Ltd
Bloomlife, Inc.
Brimrose Technology Corp
Brun Health Pvt Ltd
Children's Research Institute
Convergent Engineering, Inc.
Cornell University
Drexel University
Embryyo Technologies Pvt Ltd
Empathy Design Labs Pvt Ltd
Farus LLC
Fetal Life LLC
First Pulse Medical, Inc.
Hera Med Ltd
Imperial College London
Iris Biomedical
Kali Healthcare Pty Ltd
King Abdullah University of Science and Technology
Lifewave Biomedical Inc
Lono Medical Systems, LLC.
Marani Health Inc
MBDevice, LLC
Melody International Ltd
Monash University
Neoventa Medical AB
Noninvasix Inc
Perinatronics Medical systems Inc
Pradin Technologies Pvt Ltd
PreTel Inc
Raydiant Oximetry Inc
Rubi Life LLC
Sonica Ltd
Storx Technologies Inc
TinyKicks Inc
Tristan Technologies Inc
Two Dimensional Instruments, LLC
University of California Irvine
University of Illinois at Chicago
University of Melbourne
University of Southern California
Table of Contents
Table
Figures
Frequently asked questions
-
Which segment has the highest number of pipeline products in the Fetal Monitors pipeline market?
As of August 2023, antepartum monitors is leading in the market.
-
Which territory has the highest number of pipeline products in the Fetal Monitors pipeline market?
As of August 2023, the US has the highest number of pipeline products in the Fetal Monitors pipeline market.
-
Which is the most followed regulatory pathway in the Fetal Monitors pipeline market?
As of August 2023, 510(k) is the most followed regulatory pathway in the Fetal Monitors pipeline market.
-
Who are the major players operating in the Fetal Monitors pipeline market?
Some of the key companies in the Fetal Monitors pipeline market are Applied Physics Systems Inc., BardyDx Inc., BCB Informatica Y Control SL, Biorithm Pte Ltd, and Biotricity Inc. among others.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Fetal Monitors reports