Regulatory Approved Apps Market Size by Segments, Share, Regulatory, Reimbursement, and Forecast to 2033
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Regulatory Approved Apps Market Report Overview
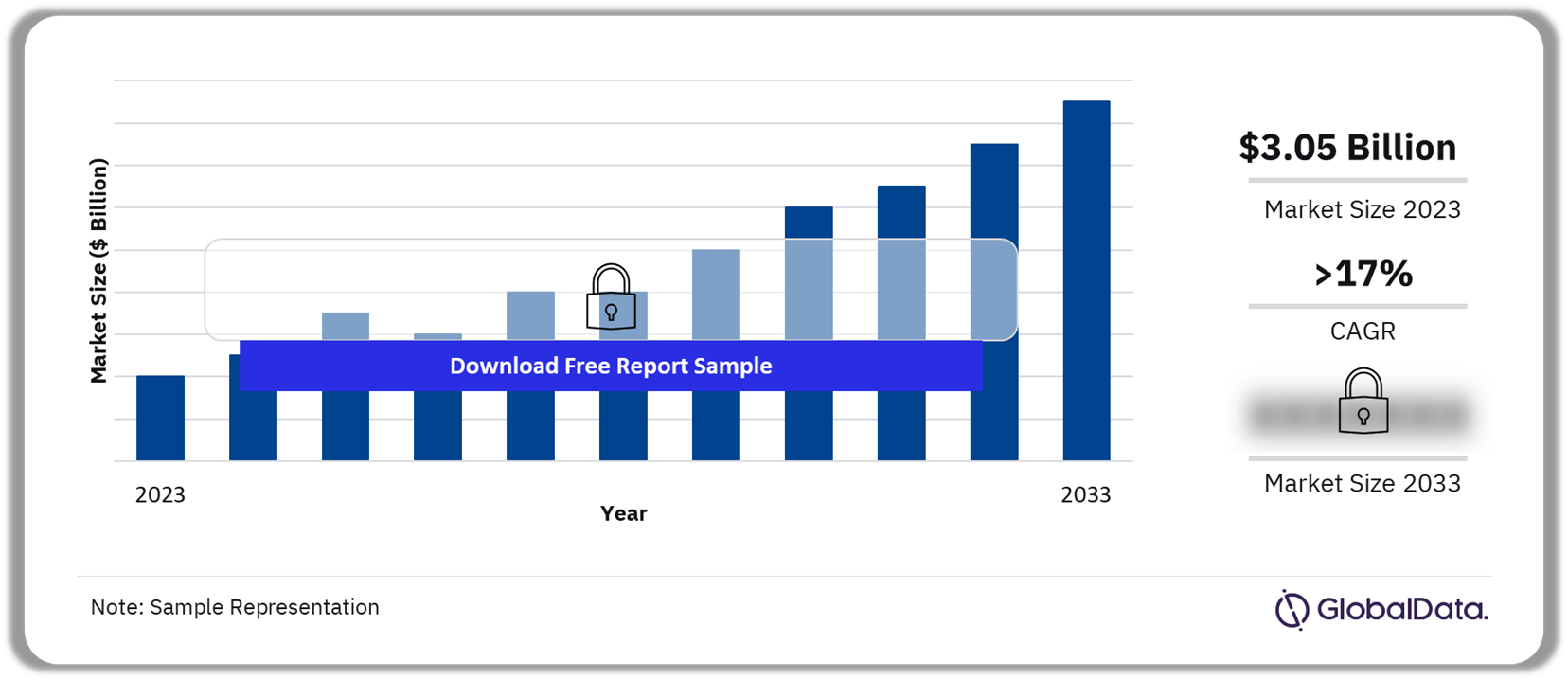
The regulatory-approved apps market size was $3.05 billion in 2023. The market will grow at a CAGR of more than 17% during 2023-2033. Regulated apps are a relatively new market being categorized as medical devices. They require a prescription to access and qualify as digital therapeutics. There is a difference in the quality of apps available as wellness aids and those that have met the regulatory burden to meet regulatory approval.
Regulatory Approved Apps Market Outlook, 2023-2033 ($ Billion)
Buy the Full Report for More Insights into the Regulatory Approved Apps Market Forecast
The regulatory approved apps market research report has been created to help understand market behavior, which will help identify quantitative market trends in the digital health therapeutic area. It discusses in detail the impact of COVID-19 on the regulatory approved apps market for the year 2020 and beyond. The report has extensively covered pipeline products and technologies, which will help in identifying companies with the most robust pipeline. This, in turn, will assist in predictive analysis for designing your in-licensing and out-licensing strategies.
| Market Size (2023) | $3.05 billion |
| CAGR (2023-2033) | >17% |
| Forecast Period | 2023-2033 |
| Historical Period | 2015-2022 |
| Key Segments | · Clinical-Focused Apps
· Indication Specific Mobile Apps |
| Key Regions | · North America
· Europe · Asia-Pacific · South and Central America · Middle East and Africa |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Regulatory Approved Apps Market Dynamics
The regulated apps market will grow during the forecast years as government regulators, medical device manufacturers, and app developers gain experience. Currently, large medical device manufacturers with digital systems have a strong competitive advantage within the market. Their familiarity with medical device classification, development, and relationship with regulators helps them have an edge. During the COVID-19 pandemic, the market did not decline as regulated apps were considered a significant tool for maintaining healthcare services and management of data.
Regulatory Approved Apps Market Segments
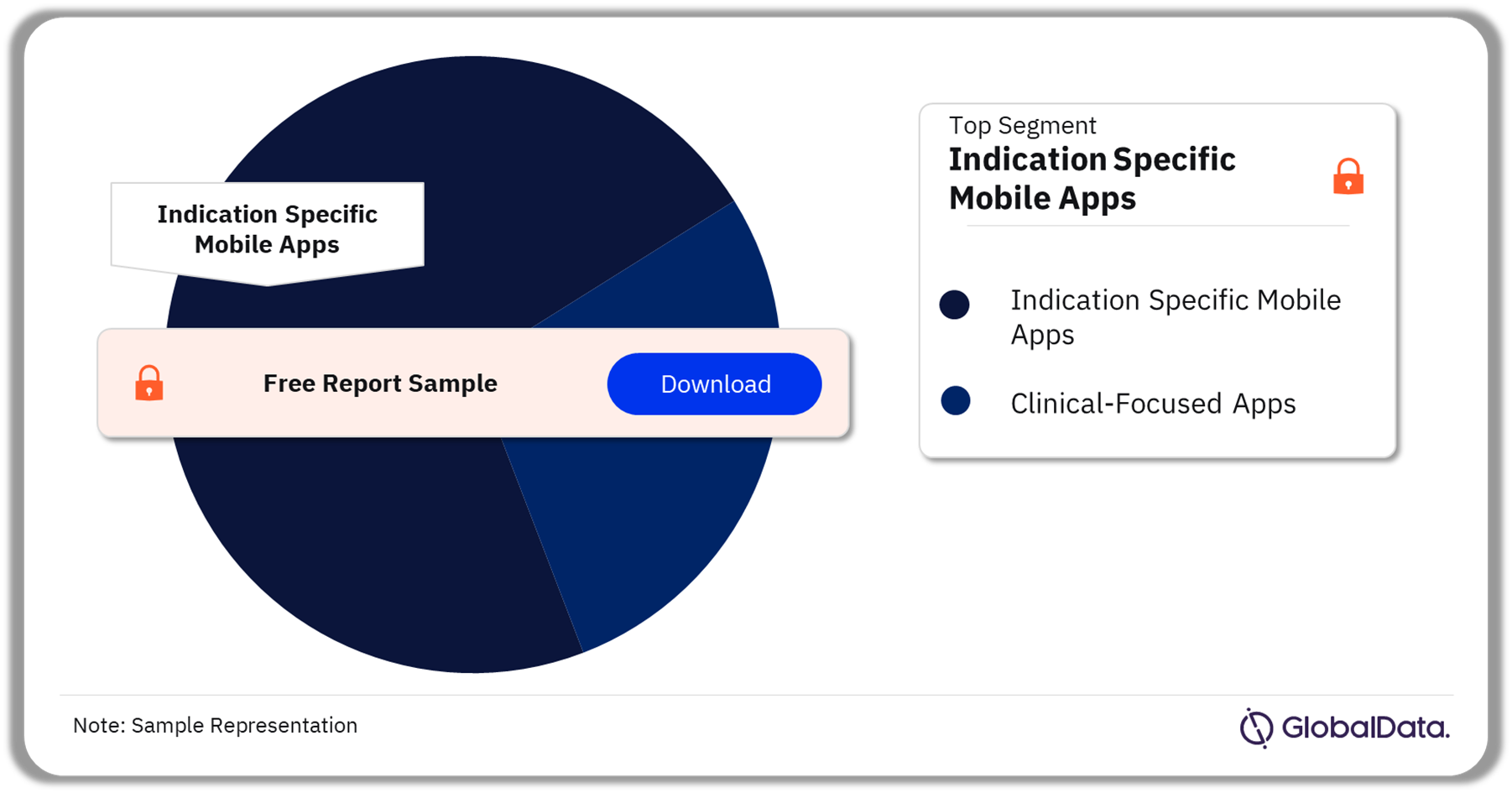
The key segments in the regulatory approved apps market are clinical-focused apps and indication-specific mobile apps. In 2023, the indication-specific mobile app dominated the market share. This app can be further categorized into cancer app, depression app, IBS app, and obesity app, among others. Such apps are made to track precise health metrics that are extremely specific for a given disease even outside of clinical settings.
Regulatory Approved Apps Market Analysis by Segments, 2023 (%)
Buy the Full Report for More Segment Insights into the Regulatory Approved Apps Market
Regulatory Approved Apps Market Segmentation by Regions
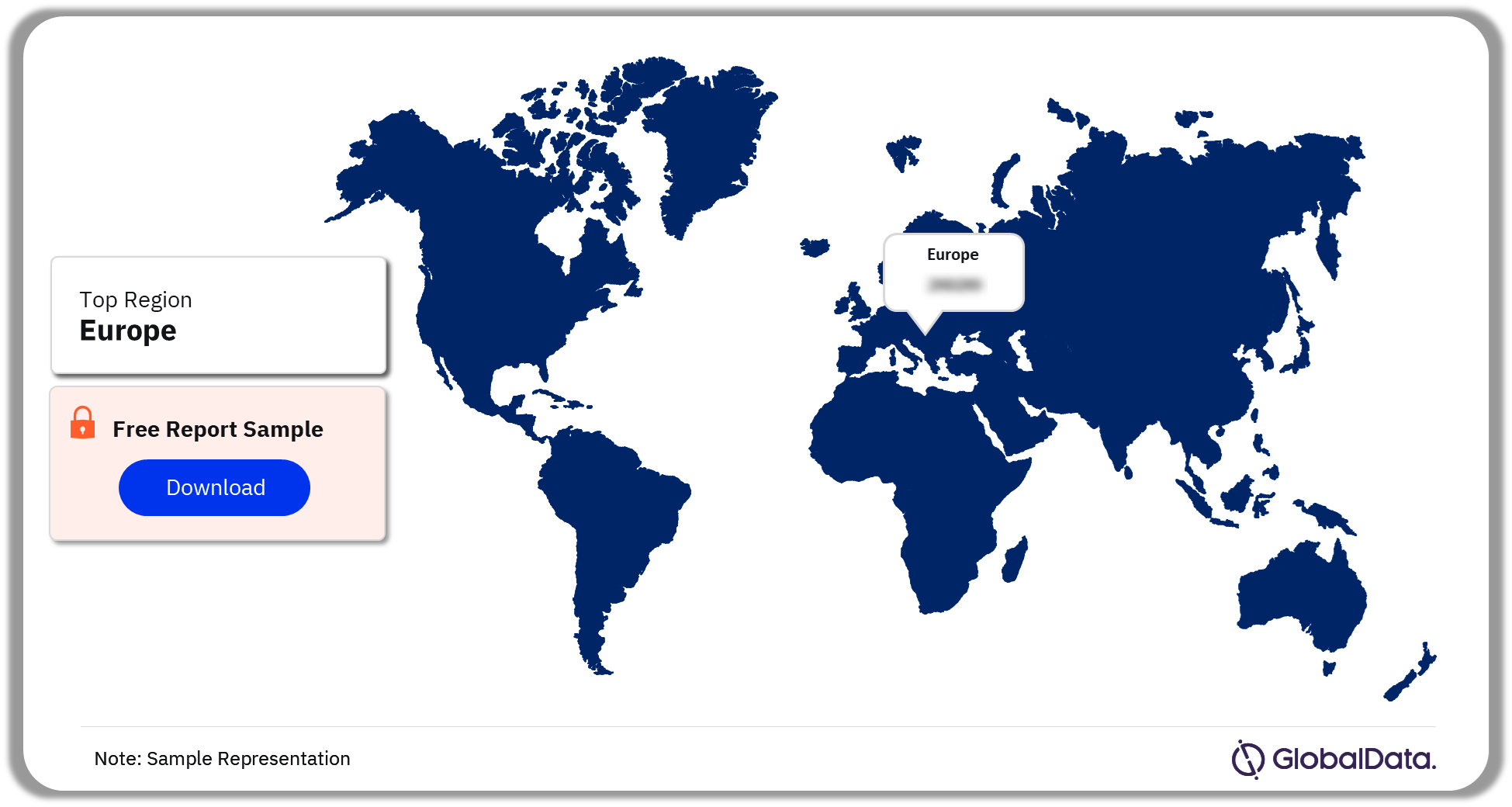
The key regions in the regulatory approved apps market are North America, Europe, Asia-Pacific, South and Central America, and the Middle East and Africa. In 2023, Europe dominated the regulatory approved apps market. The regulated apps market in Europe is one of the most dynamic and competitive in the world, with active tenders in almost every area. Increasing and rapid digitization will drive the rise in the regional market in the coming years.
Regulatory Approved Apps Market Analysis by Regions, 2023 (%)
Buy the Full Report for More Regional Insights into the Regulatory Approved Apps Market
Segments Covered in the Report
Regulatory Approved Apps Segments Outlook (Value, $ Billion, 2015-2033)
- Clinical-Focused Apps
- Indication Specific Mobile Apps
Regulatory Approved Apps Regional Outlook (Value, $ Billion, 2015-2033)
- North America
- Europe
- Asia-Pacific
- South and Central America
- Middle East & Africa
Key Inclusions
Currently marketed regulatory approved apps and evolving competitive landscape:
- Insightful review of the key industry trends.
- Annualized total Regulatory Approved Apps market revenue by segment and market outlooks from 2015-2033.
- Market-level data on units, average selling prices, and market values.
Global, Regional, and Country level market specific insights:
- Qualitative market-specific information is available with global trends further broken down into regional trends. In addition, GlobalData analysts provide unique country-specific insights on the market.
- SWOT analysis for the Regulatory Approved Apps market.
- Competitive dynamics insights and trends provided for the Regulatory Approved Apps market.
Get an in-depth understanding of consumer behavior through the market access segment and the overview of the healthcare system. The report also offers information on reimbursement policies and the regulatory landscape.
- Country-specific overview of the healthcare system.
- Country-specific reimbursement policies.
- Country-specific medtech regulatory landscape.
Robust methodologies and sources enable the model to provide an extensive and accurate overview of the market. Demand and supply-side primary sources are integrated within the syndicated models, including Key Opinion Leaders. In addition, the report helps determine market trends through real-world data sources including government procedure databases, hospital purchasing databases, and proprietary online databases.
Companies covered: Others.
Countries covered: United States, United Kingdom, Germany, France, Italy, Spain, Brazil, China, India, Russia, Japan, Australia, Canada, Mexico, South Korea, Denmark, Ireland, Netherlands, New Zealand, South Africa, Sweden, Switzerland, Austria, Belgium, Finland, Israel, Norway, Poland, Portugal, Taiwan, Czech Republic, Greece, Hungary, Turkey, Egypt, Saudi Arabia, United Arab Emirates, Argentina, and Chile.
Scope
This market model gives important, expert insight you won’t find in any other source. The model illustrates qualitative and quantitative trends within the specified market. This model is required reading for:
- CMO executives who must have a deep understanding of the regulatory approved apps marketplace to make strategic planning and investment decisions.
- Sourcing and procurement executives who must understand crucial components of the supply base to make decisions about supplier selection and management.
- Private equity investors who need a deeper understanding of the market to identify and value potential investment targets.
Reasons to Buy
- Understand the impact of COVID-19 on the regulatory approved apps market.
- Develop and design your in-licensing and out-licensing strategies through a review of pipeline products and technologies, and by identifying the companies with the most robust pipeline.
- Develop business strategies by understanding the trends shaping and driving the Regulatory Approved Apps market.
- Drive revenues by understanding the key trends, innovative products and technologies, market segments, and companies likely to impact the Regulatory Approved Apps market in the future.
- Formulate effective sales and marketing strategies by understanding the competitive landscape and by analyzing the company share of market leaders.
- Identify emerging players with potentially strong product portfolios and create effective counterstrategies to gain a competitive advantage.
- Track device sales in the global and country-specific Regulatory Approved Apps market from 2015-2033.
- Organize your sales and marketing efforts by identifying the market categories and segments that present maximum opportunities for consolidations, investments, and strategic partnerships.
Frequently asked questions
-
What was the regulatory approved apps market size in 2023?
The regulatory approved apps market size was $3.05 billion in 2023.
-
What will the regulatory approved apps market growth rate be during the forecast period?
The regulatory approved apps market is expected to grow at a CAGR of more than 17% during 2023-2033.
-
Which region dominated the regulatory approved apps market in 2023?
Europe dominated the overall regulatory approved apps market in 2023.
-
Which segment led the regulatory approved apps market in 2023?
Indication-specific mobile apps accounted for the highest share of the regulatory approved apps market in 2023.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Healthcare IT reports