Image Guided Surgery – Pipeline Products by Stage of Development 19
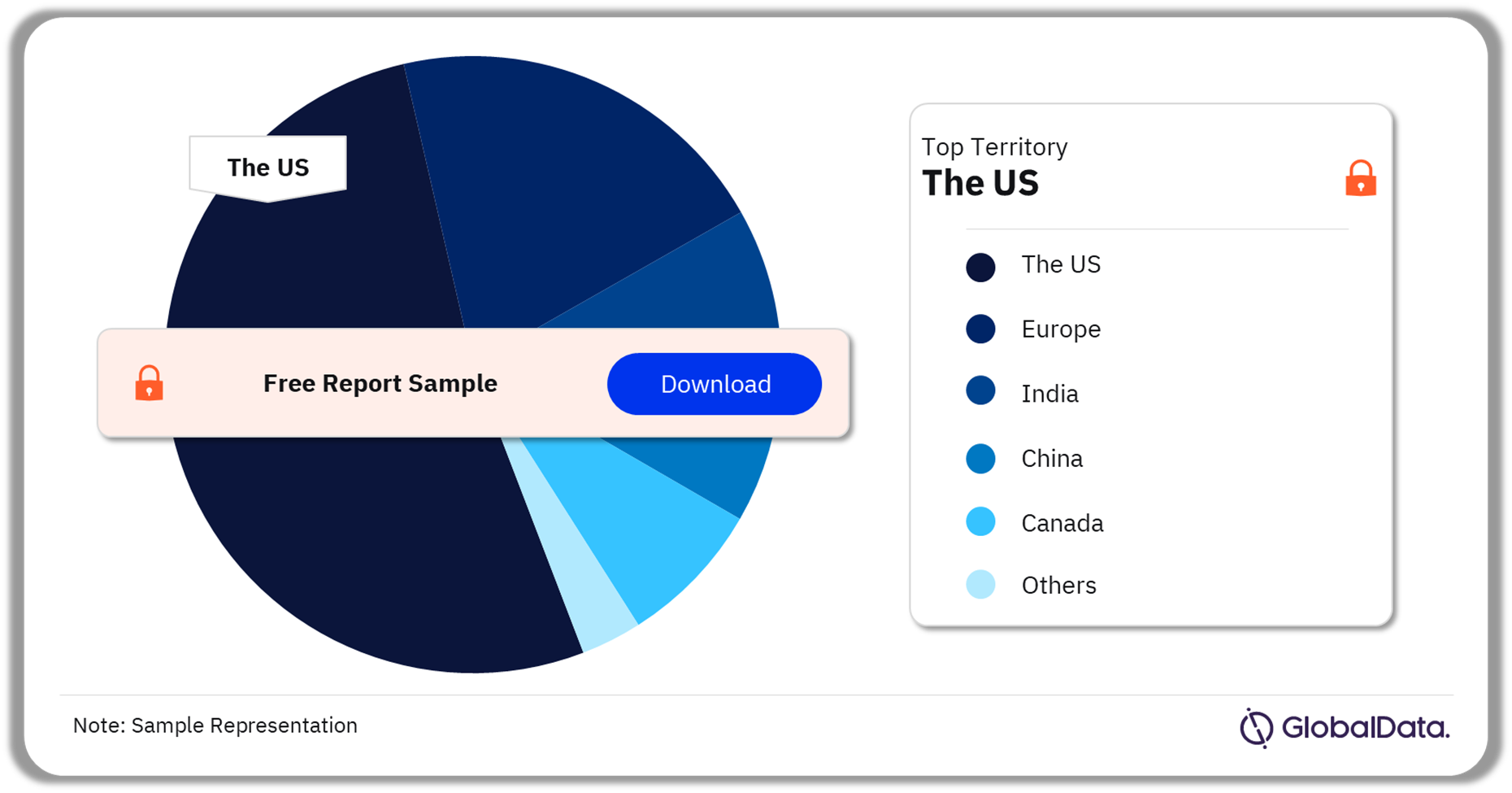
Image Guided Surgery – Pipeline Products by Territory 20
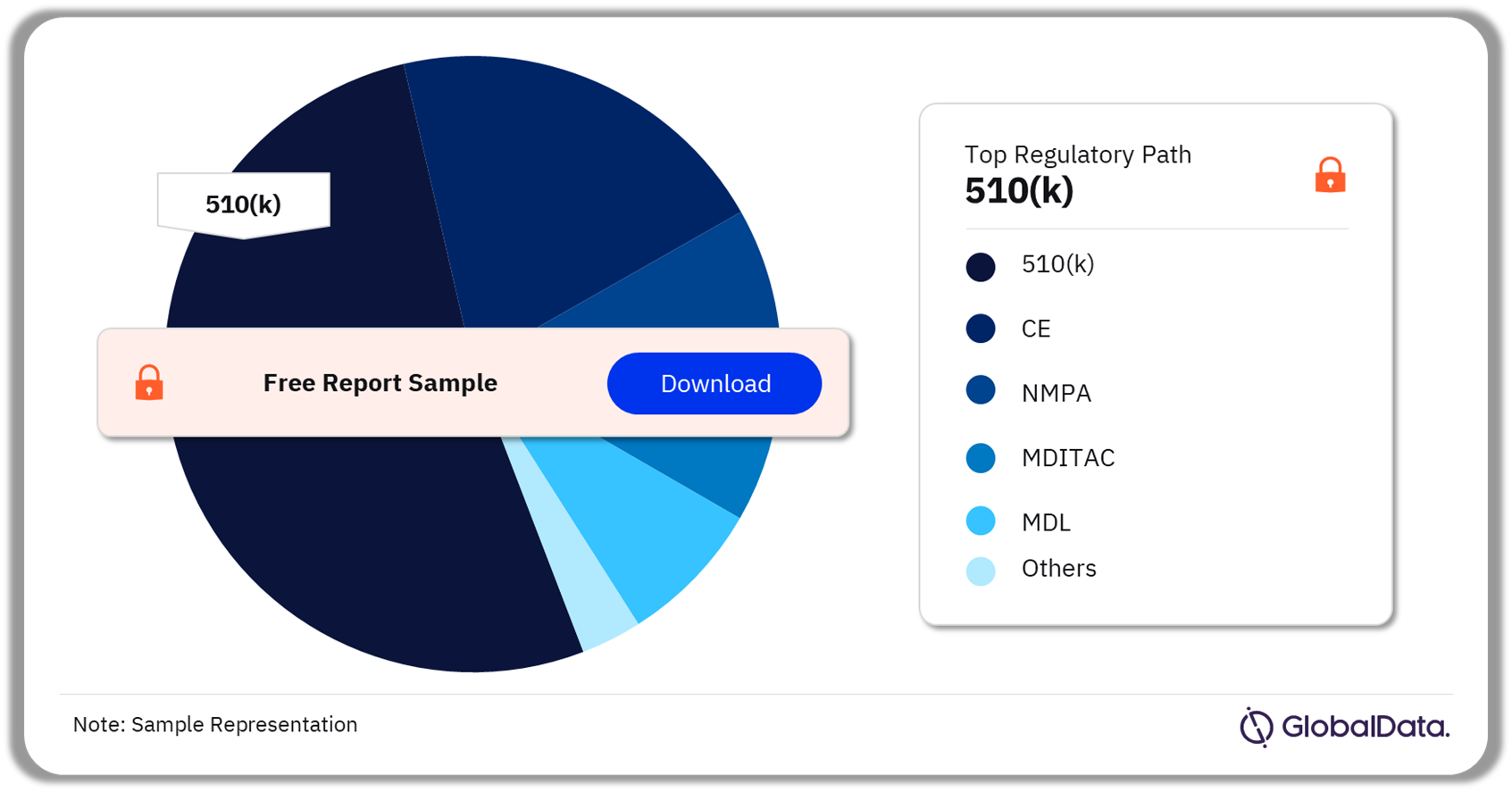
Image Guided Surgery – Pipeline Products by Regulatory Path 21
Image Guided Surgery – Pipeline Products by Estimated Approval Date 22
Image Guided Surgery – Ongoing Clinical Trials 23
Image Guided Surgery Companies – Pipeline Products by Stage of Development 24
Image Guided Surgery – Pipeline Products by Stage of Development 28
3DT Holdings LLC Pipeline Products & Ongoing Clinical Trials Overview 32
Lymphatic Access Tool Kit – Product Status 32
Lymphatic Access Tool Kit – Product Description 32
Advanced Tactile Imaging Inc Pipeline Products & Ongoing Clinical Trials Overview 33
Raman Spectroscopy Tactile Imager – Cancer – Product Status 33
Raman Spectroscopy Tactile Imager – Cancer – Product Description 33
Arspectra Sarl Pipeline Products & Ongoing Clinical Trials Overview 34
ARSpectra Device – Product Status 34
ARSpectra Device – Product Description 34
ArthroLense Inc Pipeline Products & Ongoing Clinical Trials Overview 35
AR Knee System – Product Status 35
AR Knee System – Product Description 35
Beijing Digital Precision Medicine Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 36
Fluorescence Molecular Imaging Surgical Navigation System – Product Status 36
Fluorescence Molecular Imaging Surgical Navigation System – Product Description 36
Ben-Gurion University of the Negev Pipeline Products & Ongoing Clinical Trials Overview 37
Image Guided Autonomous Needle Insertion Device – Product Status 37
Image Guided Autonomous Needle Insertion Device – Product Description 37
Beyeonics Surgical Ltd Pipeline Products & Ongoing Clinical Trials Overview 38
Beyeonics One – Product Status 38
Beyeonics One – Product Description 39
Visualization System – Brain – Product Status 39
Visualization System – Brain – Product Description 39
Visualization System – ENT – Product Status 40
Visualization System – ENT – Product Description 40
Visualization System – Plastic Surgery – Product Status 40
Visualization System – Plastic Surgery – Product Description 41
Visualization System – Spine – Product Status 41
Visualization System – Spine – Product Description 41
BioOptico AB Pipeline Products & Ongoing Clinical Trials Overview 42
Augmented Arthroscopy System – Product Status 42
Augmented Arthroscopy System – Product Description 42
Augmented Reality Tool – Endoscopy – Product Status 43
Augmented Reality Tool – Endoscopy – Product Description 43
BrainLAB AG Pipeline Products & Ongoing Clinical Trials Overview 44
Curve – WLAN Module – Product Status 44
Curve – WLAN Module – Product Description 44
Kick EM – Product Status 45
Kick EM – Product Description 45
Brigham and Women’s Hospital Pipeline Products & Ongoing Clinical Trials Overview 46
Stimulated Raman Imaging Device – Brain Tumor Surgery – Product Status 46
Stimulated Raman Imaging Device – Brain Tumor Surgery – Product Description 46
Carbon (Shenzhen) Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 47
Fusion Surgery System – Product Status 47
Fusion Surgery System – Product Description 47
Cartosense Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 48
Iris Surgical Navigation System – Product Status 48
Iris Surgical Navigation System – Product Description 48
Centerline Biomedical Inc Pipeline Products & Ongoing Clinical Trials Overview 49
HoloLens AR based Intra-Operative Positioning System – Product Status 49
HoloLens AR based Intra-Operative Positioning System – Product Description 49
Intra-Operative Positioning System (iOPS) – Product Status 50
Intra-Operative Positioning System (iOPS) – Product Description 50
Centerline Biomedical Inc – Ongoing Clinical Trials Overview 51
Intra-Operative Positioning System (iOPS) – 3D Holographic Guidance, Navigation, and Control (3D GN&C) for Endovascular Aortic Repair (EVAR) 52
Intra-Operative Positioning System (iOPS) – A Study to Clinically Evaluate the Effectiveness and Safety of the Technology in Providing Accurate Navigation During Endovascular Aortic Repair (EVAR) Procedures 52
Changzhou Jishuo Medical Equipment Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 53
Mixed Reality Knee Surgery Navigation System – Product Status 53
Mixed Reality Knee Surgery Navigation System – Product Description 53
Mixed Reality Lung Navigation System – Product Status 54
Mixed Reality Lung Navigation System – Product Description 54
Clear Guide Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 55
Augmented Reality Device – Spine Surgery – Product Status 55
Augmented Reality Device – Spine Surgery – Product Description 55
Clear Guide Scenergy – Pediatric – Product Status 56
Clear Guide Scenergy – Pediatric – Product Description 56
ColdSteel Laser Inc Pipeline Products & Ongoing Clinical Trials Overview 57
Remote Image-Guided Endoscopic Laser Surgery (RIGES) Device – Product Status 57
Remote Image-Guided Endoscopic Laser Surgery (RIGES) Device – Product Description 57
cortEXplore GmbH Pipeline Products & Ongoing Clinical Trials Overview 58
CORTEXPLORER MED System – Product Status 58
CORTEXPLORER MED System – Product Description 58
Creo Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 59
Super-Cable Device – Product Status 59
Super-Cable Device – Product Description 59
Crystalline Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 60
Ultrasound-Guided Pericardial Access Device – Product Status 60
Ultrasound-Guided Pericardial Access Device – Product Description 60
Dartmouth College Pipeline Products & Ongoing Clinical Trials Overview 61
Image Guided Surgery – Calibrated System – Product Status 61
Image Guided Surgery – Calibrated System – Product Description 61
Intraoperative Stereovision System (iSV) – Product Status 62
Intraoperative Stereovision System (iSV) – Product Description 62
Delft University of Technology Pipeline Products & Ongoing Clinical Trials Overview 63
Endovascular Instrument – Product Status 63
Endovascular Instrument – Product Description 63
EchoGuide BV Pipeline Products & Ongoing Clinical Trials Overview 64
EchoGuide – Product Status 64
EchoGuide – Product Description 64
fiagon GmbH Pipeline Products & Ongoing Clinical Trials Overview 65
Cube Navigation System – Product Status 65
Cube Navigation System – Product Description 65
VirtuEye Gen2 – Product Status 66
VirtuEye Gen2 – Product Description 66
Florida International University Pipeline Products & Ongoing Clinical Trials Overview 67
Intraoperative Guidance System for Tumor Surgery – Product Status 67
Intraoperative Guidance System for Tumor Surgery – Product Description 67
FUJIFILM Medical Systems USA Inc Pipeline Products & Ongoing Clinical Trials Overview 68
Endosurgical Image Enhancement System – Product Status 68
Endosurgical Image Enhancement System – Product Description 68
GE HealthCare Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 69
Image Guided Surgical System – Product Status 69
Image Guided Surgical System – Product Description 69
GE Medical Systems SCS Pipeline Products & Ongoing Clinical Trials Overview 70
Allia IGS 5 – Product Status 70
Allia IGS 5 – Product Description 70
Gentuity LLC Pipeline Products & Ongoing Clinical Trials Overview 71
Vis-M – Product Status 71
Vis-M – Product Description 71
Vis-N Device – Product Status 72
Vis-N Device – Product Description 72
Gentuity LLC – Ongoing Clinical Trials Overview 73
Vis-M – Clinical Feasibility Evaluation of the Gentuity HF-OCT Imaging System With Vis-M Micro-Imaging Catheter 74
German Cancer Research Center Pipeline Products & Ongoing Clinical Trials Overview 75
Fluorescence Guided Surgery System – Product Status 75
Fluorescence Guided Surgery System – Product Description 75
GYS Tech LLC Pipeline Products & Ongoing Clinical Trials Overview 76
Surgical Navigation System – Spine – Product Status 76
Surgical Navigation System – Spine – Product Description 76
HoloSurgical Inc Pipeline Products & Ongoing Clinical Trials Overview 77
ARAI Surgical Navigation System – General Surgery – Product Status 77
ARAI Surgical Navigation System – General Surgery – Product Description 77
IGI Technologies, Inc. Pipeline Products & Ongoing Clinical Trials Overview 78
LapAR Visualization System – Product Status 78
LapAR Visualization System – Product Description 78
Imperial College London Pipeline Products & Ongoing Clinical Trials Overview 79
Prostate Cancer-Detecting Probe – Product Status 79
Prostate Cancer-Detecting Probe – Product Description 79
IMRIS, Inc. Pipeline Products & Ongoing Clinical Trials Overview 80
Image Guided Radiation Therapy System – Brain Cancer – Product Status 80
Image Guided Radiation Therapy System – Brain Cancer – Product Description 80
Insight Medical Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 81
ARVIS – Musculoskeletal Oncology – Product Status 81
ARVIS – Musculoskeletal Oncology – Product Description 81
iSYS Medizintechnik GmbH Pipeline Products & Ongoing Clinical Trials Overview 82
Micromate – New Version – Product Status 82
Micromate – New Version – Product Description 82
Johns Hopkins University Pipeline Products & Ongoing Clinical Trials Overview 83
3D Virtual Simulation Guided Ablation Device – Product Status 83
3D Virtual Simulation Guided Ablation Device – Product Description 83
Fluoro-Servoing Robotic System – Product Status 84
Fluoro-Servoing Robotic System – Product Description 84
Photoacoustic Imaging – Transsphenoidal Surgery – Product Status 84
Photoacoustic Imaging – Transsphenoidal Surgery – Product Description 85
King’s College London Pipeline Products & Ongoing Clinical Trials Overview 86
Interventional Magnetic Resonance Imaging Tool – Product Status 86
Interventional Magnetic Resonance Imaging Tool – Product Description 86
Kitware Inc Pipeline Products & Ongoing Clinical Trials Overview 87
iCSPlan – Product Status 87
iCSPlan – Product Description 87
Koninklijke Philips NV Pipeline Products & Ongoing Clinical Trials Overview 88
ClarifEye Augmented Reality Surgical Navigation System – Product Status 88
ClarifEye Augmented Reality Surgical Navigation System – Product Description 89
Kornerstone Devices Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 90
HighNoon – Product Status 90
HighNoon – Product Description 90
LumaMed LLC Pipeline Products & Ongoing Clinical Trials Overview 91
LumaScan II – Product Status 91
LumaScan II – Product Description 91
LumaScan M – Product Status 92
LumaScan M – Product Description 92
Lumos, Inc. Pipeline Products & Ongoing Clinical Trials Overview 93
Lumos RTV System – Product Status 93
Lumos RTV System – Product Description 93
Mariner Endosurgery Inc Pipeline Products & Ongoing Clinical Trials Overview 94
Phylax – Product Status 94
Phylax – Product Description 94
Massachusetts Institute of Technology Pipeline Products & Ongoing Clinical Trials Overview 95
Image-Guided Surgical System – Ovarian Cancer – Product Status 95
Image-Guided Surgical System – Ovarian Cancer – Product Description 95
MediView XR Inc Pipeline Products & Ongoing Clinical Trials Overview 96
Acuity System – Kidney Tumour Ablation – Product Status 96
Acuity System – Kidney Tumour Ablation – Product Description 96
RTFHV Extended Reality Surgical Navigation System – Product Status 97
RTFHV Extended Reality Surgical Navigation System – Product Description 97
XR50 – Product Status 97
XR50 – Product Description 98
Medulla Pro Technology Pte Ltd Pipeline Products & Ongoing Clinical Trials Overview 99
Ultrasound-Guided Device – Lumbar Puncture – Product Status 99
Ultrasound-Guided Device – Lumbar Puncture – Product Description 99
Moor Instruments Ltd Pipeline Products & Ongoing Clinical Trials Overview 100
Keyhole Surgery Imaging Device – Product Status 100
Keyhole Surgery Imaging Device – Product Description 100
Navigation Sciences Inc Pipeline Products & Ongoing Clinical Trials Overview 101
NaviSci-EndoMarker – Product Status 101
NaviSci-EndoMarker – Product Description 101
Navigation Sciences Inc – Ongoing Clinical Trials Overview 102
NaviSci-EndoMarker – Image – Navigated Resection of Lung Nodules 103
NDR Medical Technology Pte Ltd Pipeline Products & Ongoing Clinical Trials Overview 104
ANT-X System – Product Status 104
ANT-X System – Product Description 104
Automated Needle Targeting System (ANT-C) – Lung Biopsy – Product Status 105
Automated Needle Targeting System (ANT-C) – Lung Biopsy – Product Description 105
NDR Medical Technology Pte Ltd – Ongoing Clinical Trials Overview 106
ANT-X System – A Randomized Controlled Trial to Evaluate the Efficacy and Safety of Automated Needle Targeting (ANT-X) System Compared to Traditional Free Hand Puncture for Renal Access in Percutaneous Nephrolithotomy (PCNL) Performed by Urologists in Training 107
NuVasive Inc Pipeline Products & Ongoing Clinical Trials Overview 108
NuVasive Pulse System – 2D Navigation – Product Status 108
NuVasive Pulse System – 2D Navigation – Product Description 108
NuVasive Pulse System – LessRay – Product Status 109
NuVasive Pulse System – LessRay – Product Description 109
Ocutrx Vision Technologies LLC Pipeline Products & Ongoing Clinical Trials Overview 110
DigiLoupe AR/XR Headset – Product Status 110
DigiLoupe AR/XR Headset – Product Description 110
OncoRes Medical Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 111
Intraoperative Imaging Device – Product Status 111
Intraoperative Imaging Device – Product Description 111
OnLume Inc Pipeline Products & Ongoing Clinical Trials Overview 112
Wide-Field FGS Imaging System – Product Status 112
Wide-Field FGS Imaging System – Product Description 112
Palion Medical AS Pipeline Products & Ongoing Clinical Trials Overview 113
MultiGuide – Product Status 113
MultiGuide – Product Description 113
Palion Medical AS – Ongoing Clinical Trials Overview 114
MultiGuide – Botulinum Toxin Type A Blockade of the Sphenopalatine Ganglion in Treatment-refractory Chronic Cluster Headache 115
MultiGuide – Botulinum Toxin Type A Blockade of the Sphenopalatine Ganglion in Treatment-refractory Chronic Migraine 115
Pathfinder Technologies (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 116
Image-Guided Surgery System – Kidney Surgery – Product Status 116
Image-Guided Surgery System – Kidney Surgery – Product Description 116
Surgical Instrument Localization Apparatus – Product Status 117
Surgical Instrument Localization Apparatus – Product Description 117
Perimeter Medical Imaging Inc Pipeline Products & Ongoing Clinical Trials Overview 118
Otis Wide Field OCT – Head And Neck Cancer – Product Status 118
Otis Wide Field OCT – Head And Neck Cancer – Product Description 118
Pixee Medical Pipeline Products & Ongoing Clinical Trials Overview 119
Shoulder Implant Navigation System – Product Status 119
Shoulder Implant Navigation System – Product Description 119
Promaxo Inc Pipeline Products & Ongoing Clinical Trials Overview 120
PROMAXO MRI Guided Therapy Device – Product Status 120
PROMAXO MRI Guided Therapy Device – Product Description 120
Raydiax GmbH Pipeline Products & Ongoing Clinical Trials Overview 121
TACT – Product Status 121
TACT – Product Description 121
Rivanna Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 122
3D Spinal Navigation System – Product Status 122
3D Spinal Navigation System – Product Description 122
Handheld Ultrasound System – Thoracic Epidural Guidance – Product Status 123
Handheld Ultrasound System – Thoracic Epidural Guidance – Product Description 123
Robarts Research Institute Pipeline Products & Ongoing Clinical Trials Overview 124
360-Degree 3D Ultrasound System – Product Status 124
360-Degree 3D Ultrasound System – Product Description 124
Robin Medical, Inc. Pipeline Products & Ongoing Clinical Trials Overview 125
MRI Guided RFA Probe – Product Status 125
MRI Guided RFA Probe – Product Description 125
SG Devices LLC Pipeline Products & Ongoing Clinical Trials Overview 126
Mixed-Reality Surgical System – Product Status 126
Mixed-Reality Surgical System – Product Description 126
SG Devices LLC – Ongoing Clinical Trials Overview 127
Mixed-Reality Surgical System – Novel Wireless Mixed Reality Headset for Image Guidance in Cardiac Catheterization Laboratory 128
Shanghai Linyan Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 129
AR Surgical Navigation System – Joint Surgery – Product Status 129
AR Surgical Navigation System – Joint Surgery – Product Description 129
AR Surgical Navigation System – Neurosurgery – Product Status 130
AR Surgical Navigation System – Neurosurgery – Product Description 130
AR Surgical Navigation System – Pulmonary Surgery – Product Status 130
AR Surgical Navigation System – Pulmonary Surgery – Product Description 131
AR Surgical Navigation System – Sports Medicine – Product Status 131
AR Surgical Navigation System – Sports Medicine – Product Description 131
Holonavi S – Product Status 132
Holonavi S – Product Description 132
Siemens Healthineers AG Pipeline Products & Ongoing Clinical Trials Overview 133
Intra-Operative 3D-Imaging And Navigation Tool – Product Status 133
Intra-Operative 3D-Imaging And Navigation Tool – Product Description 133
Simm High-Tech (Jiangsu) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 134
Intraoperative Imaging Device – Product Status 134
Intraoperative Imaging Device – Product Description 134
Simm High-Tech (Jiangsu) Co Ltd – Ongoing Clinical Trials Overview 135
Intraoperative Imaging Device – A Multi-Centre Clinical Trial Study Evaluating the Safety and Functionality of the ORM-P2 System for Differentiating Tissue Types in the Resection Bed During Breast-conserving Surgery 136
Sonavex Inc Pipeline Products & Ongoing Clinical Trials Overview 137
EchoGuide – Product Status 137
EchoGuide – Product Description 137
South African Medical Research Council Pipeline Products & Ongoing Clinical Trials Overview 138
Image-guided Surgical Navigator – Product Status 138
Image-guided Surgical Navigator – Product Description 138
Southern Illinois University Carbondale Pipeline Products & Ongoing Clinical Trials Overview 139
Ultrasonic 3D Navigation System – Product Status 139
Ultrasonic 3D Navigation System – Product Description 139
Spectropath Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 140
Spectropath Image-Guided Surgery System – Product Status 140
Spectropath Image-Guided Surgery System – Product Description 140
Spectropath System – Skin Cancer Screening – Product Status 141
Spectropath System – Skin Cancer Screening – Product Description 141
Spectropath System With CT – Product Status 141
Spectropath System With CT – Product Description 142
Spectropath System With MR – Product Status 142
Spectropath System With MR – Product Description 142
Spectropath System With Robotic Surgery – Product Status 143
Spectropath System With Robotic Surgery – Product Description 143
Stanford University Pipeline Products & Ongoing Clinical Trials Overview 144
MR Guided Focused Ultrasound Surgery – Product Status 144
MR Guided Focused Ultrasound Surgery – Product Description 144
SurgVision BV Pipeline Products & Ongoing Clinical Trials Overview 145
MetVision – Product Status 145
MetVision – Product Description 145
Suzhou Guoke Kangcheng Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 146
Kangcheng Ruihang – Product Status 146
Kangcheng Ruihang – Product Description 146
Tel Aviv University Pipeline Products & Ongoing Clinical Trials Overview 147
Smart Probe – Product Status 147
Smart Probe – Product Description 147
The Acrobot Company Limited (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 148
Acrobot Navigator System – Product Status 148
Acrobot Navigator System – Product Description 148
The Methodist Hospital Research Institute Pipeline Products & Ongoing Clinical Trials Overview 149
Molecular Image Guided System – Product Status 149
Molecular Image Guided System – Product Description 149
TherVoyant Inc Pipeline Products & Ongoing Clinical Trials Overview 150
GuideRT – Product Status 150
GuideRT – Product Description 150
Unify Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 151
Fluorescence Guided Surgical Device – Pancreatic Cancer – Product Status 151
Fluorescence Guided Surgical Device – Pancreatic Cancer – Product Description 151
University Health Network Pipeline Products & Ongoing Clinical Trials Overview 152
GTx-oscope – Product Status 152
GTx-oscope – Product Description 152
University of Arizona Pipeline Products & Ongoing Clinical Trials Overview 153
Stereoscopic Augmented Microscope – Product Status 153
Stereoscopic Augmented Microscope – Product Description 153
University of Bern Pipeline Products & Ongoing Clinical Trials Overview 154
Stereotactic Instrument Guidance System – Liver Surgery – Product Status 154
Stereotactic Instrument Guidance System – Liver Surgery – Product Description 154
University of California Los Angeles Pipeline Products & Ongoing Clinical Trials Overview 155
Image-Guided Irrigating Suction Cannula – Product Status 155
Image-Guided Irrigating Suction Cannula – Product Description 155
University of California San Diego Pipeline Products & Ongoing Clinical Trials Overview 156
Dual Reflectance-Fluorescence Guided Surgical System – Product Status 156
Dual Reflectance-Fluorescence Guided Surgical System – Product Description 156
University of Florence Pipeline Products & Ongoing Clinical Trials Overview 157
HyperProbe System – Product Status 157
HyperProbe System – Product Description 157
University of Illinois at Urbana-Champaign Pipeline Products & Ongoing Clinical Trials Overview 158
Fluorescent Based – Camera – Product Status 158
Fluorescent Based – Camera – Product Description 158
University of Lille Nord de France Pipeline Products & Ongoing Clinical Trials Overview 159
SpiderMass – Product Status 159
SpiderMass – Product Description 159
University of Lille Nord de France – Ongoing Clinical Trials Overview 160
SpiderMass – Using the Spidermass for in Vivo and Real Time Mass Spectrometry Analysis of Subject’s Epidermis Suffering from Chronic Inflammatory Dermatosis Compared to Control Groups 161
University of South Florida Pipeline Products & Ongoing Clinical Trials Overview 162
Augmented Reality System – Product Status 162
Augmented Reality System – Product Description 162
University of Utah Pipeline Products & Ongoing Clinical Trials Overview 163
Catheter Tip Monitoring System – Product Status 163
Catheter Tip Monitoring System – Product Description 163
University of Washington Pipeline Products & Ongoing Clinical Trials Overview 164
Laparoscopic HIFU Clamp – Partial Nephrectomies – Product Status 164
Laparoscopic HIFU Clamp – Partial Nephrectomies – Product Description 164
Vanderbilt University Pipeline Products & Ongoing Clinical Trials Overview 165
C-in Device – Product Status 165
C-in Device – Product Description 165
Image Guidance System – Breast Cancer Surgery – Product Status 166
Image Guidance System – Breast Cancer Surgery – Product Description 166
Image Guided Navigation System – Endoscopic Eye Surgery – Product Status 166
Image Guided Navigation System – Endoscopic Eye Surgery – Product Description 167
Laser Range Scanning Device – Product Status 167
Laser Range Scanning Device – Product Description 167
Vibronix Inc. Pipeline Products & Ongoing Clinical Trials Overview 168
Acoustar – Product Status 168
Acoustar – Product Description 168
VPIX Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 169
Auto-Stage – Product Status 169
Auto-Stage – Product Description 169
Pixection – In vivo – Angled Type – Product Status 170
Pixection – In vivo – Angled Type – Product Description 170
Pixection – In vivo – Lung – Product Status 170
Pixection – In vivo – Lung – Product Description 171
Pixection – In vivo – Pen Type – Product Status 171
Pixection – In vivo – Pen Type – Product Description 171
Pixection – In vivo – Robotic Surgery – Product Status 172
Pixection – In vivo – Robotic Surgery – Product Description 172
Vuze Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 173
VUZE 2.0 – Product Status 173
VUZE 2.0 – Product Description 173
Vuzix Corp Pipeline Products & Ongoing Clinical Trials Overview 174
Head Mounted Display System – Product Status 174
Head Mounted Display System – Product Description 174
Xoran Technologies LLC Pipeline Products & Ongoing Clinical Trials Overview 175
Intraoperative CT imaging Device – Spine – Product Status 175
Intraoperative CT imaging Device – Spine – Product Description 175
xCAT IQ Integrated Surgical Navigation System – Product Status 176
xCAT IQ Integrated Surgical Navigation System – Product Description 176
Glossary 221
![]()