Likelihood of Approval and Phase Transition Success Rate Model – Lenvatinib Mesylate in Metastatic Melanoma
Powered by ![]()
Unlock hidden opportunities in the LoA industry
Empower your strategies with our Likelihood of Approval and Phase Transition Success Rate Model – Lenvatinib Mesylate in Metastatic Melanoma report and make more profitable business decisions.
This report provides you with the data that allows you to track and predict the specific likelihood of approval (LOA) and phase transition success rate (PTSR) of a drug using GlobalData’s proprietary machine learning algorithms developed using over 10 years of historical data.
Lenvatinib Mesylate in Metastatic Melanoma Drug Details:
Lenvatinib mesylate (Lenvima / Kisplyx / Lenvixi) is a pyrrolopyrimidine derivative acts as anti-neoplastic agent. It is formulated as hard gelatin capsules for oral route of administration. Lenvima is indicated for the treatment of patients with locally recurrent or metastatic, progressive, radioactive iodine-refractory differentiated thyroid cancer (DTC). Lenvima is indicated for the treatment of adult patients with progressive, locally advanced or metastatic, differentioated (papillary, follicular, Hurthel cell) thyroid carcinoma refractory to radioactive iodine and Kisplyx is indicated in combination with everolimus for the treatment of patients with advanced renal cell carcinoma following one prior anti-angiogenic therapy and for the treatment of adult patients with advanced renal cell carcinoma following one prior vascular endothelial growth factor (VEGF) targeted therapy and indicated for the first-line treatment of patients with unresectable hepatocellular carcinoma (HCC). Lenvima is indicated for the treatment of unresectable thymic carcinoma and also indicated in combination with pembrolizumab for the first-line treatment of adult patients with advanced renal cell carcinoma.It is also under development for the treatment of advanced adrenocortical cancer, malignant pleural mesothelioma, advanced/metastatic gastric cancer, metastatic esophageal squamous cell carcinoma, gastroesophageal junction adenocarcinoma, melanoma, oropharynx, hypopharynx, and/or larynx, locally advanced or metastatic gastrointestinal Stromal Tumor (GIST), neuroendocrine carcinoma, pediatric diffuse intrinsic pontine glioma, recurrent or metastaticsalivary gland cancers, anaplastic thyroid cancer, merkel cell carcinoma, pancreatic ductal adenocarcinoma, relapsed and refractory osteosarcoma, Ewing sarcoma, rhabdomyosarcoma, medulloblastoma, ependymoma, high grade glioma, breast cancer, triple-negative breast cancer, endometrial cancer, renal cell carcinoma (second line), hepatocellular carcinoma, bladder cancer, locally advanced or metastatic non-squamous non-small cell lung cancer, endometrial cancer, urothelial cancer, biliary tract cancer, advanced gastric cancer, adenoid cystic carcinoma, squamous cell carcinoma of the head and neck, non-small cell lung cancer (RET translocations), thymic carcinoma, endometrial cancer, fallopian tube cancer, peritoneal cancer, recurrent head and neck cancer squamous cell carcinoma, colorectal cancer, cutaneous squamous cell carcinoma and epithelial ovarian cancer, ampullary carcinoma, gallbladder carcinoma, prostate cancer, HER2 negative breast cancer, human papillomavirus (HPV)-associated recurrent respiratory papillomatosis (RRP) and leptomeningeal metastases from any solid tumor and mixed carcinoma. It is also under development for solid tumors in combination with pembrolizumab and for advanced or metastatic non clear cell renal cell carcinoma who have not received any chemotherapy for advanced disease. It was also under development for the treatment of recurrent or metastatic head and neck squamous cell carcinoma of oral cavity, metastatic urothelial carcinoma, lung adenocarcinoma, recurrent malignant glioma, liver fibrosis, endometrial cancer (monotherapy), melanoma and recurrent glioblastoma, metastatic melanoma, peripheral primitive neuroectodermal tumor (pPNET) and non-small cell lung cancer (third-line, monotherapy).
Report Coverage
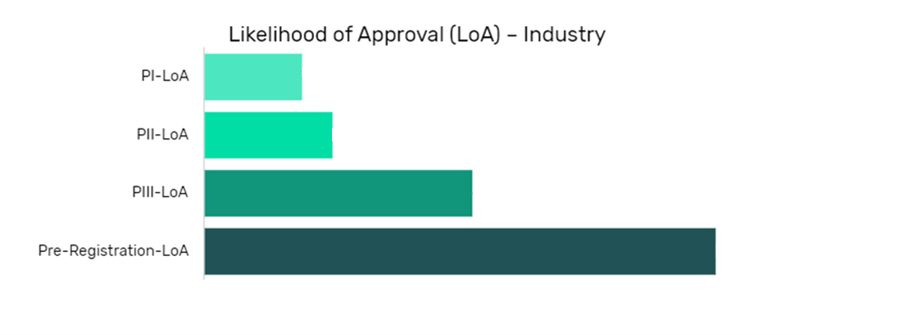
The data is segmented by drug name per indication and shows the current likelihood of approval for the drug compared to the indication benchmark and the industry benchmark.
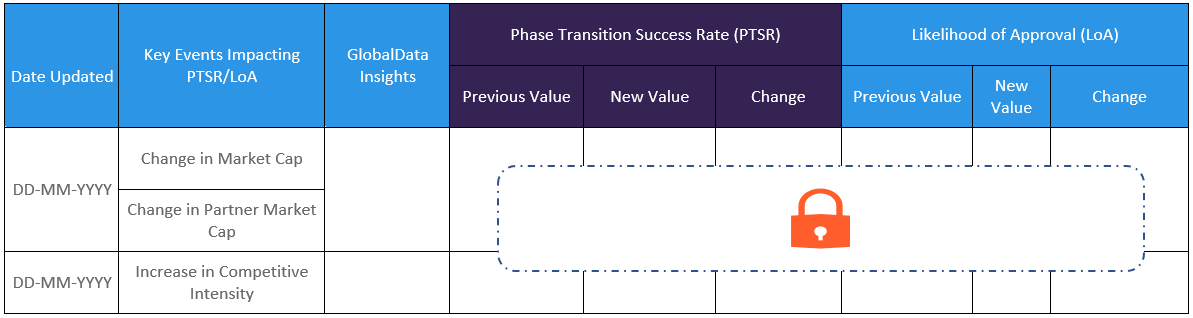
The Likelihood of Approval data is updated regularly based on events that take place which impact the clinical development process and regulatory considerations. GlobalData’s proprietary machine learning models consider these events in real time, to produce quantitative changes to the LOA and PTSR along with qualitative reasoning why the likelihood of approval has changed.
| Quick View – Lenvatinib Mesylate LOA Data | |||||
| Report Segments |
|
||||
| Drug Name |
|
||||
| Administration Pathway |
|
||||
| Therapeutic Areas |
|
||||
| Key Manufacturers |
|
||||
| Drug Development Status |
|
||||
Reasons to Buy
- Precise Likelihood of Approval and Phase Transition Success Rates: Our machine learning and proprietary models provide accurate predictions, helping you gauge the potential success of a drug in the regulatory process.
- Competitive Strategy Planning: Access information on LOA and PTSR for competitors’ drugs, allowing you to plan your clinical development, commercialisation and marketing strategies
- Event-driven Updates: Track event-driven changes in LOA and PTSR benchmarked against indication LOA/PTSR. Get the latest insights to adapt your strategies promptly!
- Well-informed Investment Decisions: This data helps you navigate the dynamic landscape of drug development and regulatory considerations.
Scope
- Drug Details: Drug name, Drug type, Intervention type
- Administration Pathway
- Therapeutic Areas
- Key Manufacturers
- Drug Development Status
This is an on-demand report that will be delivered upon request. The report will be delivered within 2 business days of the purchase, excluding weekends and holidays. Certain sections of the report may be removed or altered based on data availability and relevance.
Frequently asked questions
- Drugs which have been approved in the past 10 years
- Drugs which have failed during clinical development in the past 18 years
- Drugs which are currently in development
- Phase I, Phase II, Phase III, and Pre-Registration development stage
- Drugs must meet one of the following criteria to be included in the model:
- The developer has specified the US as an intended market for approval.
- The developer has not specified any country as an intended market for approval, i.e. the “Drug Geography” is listed as “Global”
- Innovator drugs and biosimilars
- Diagnostics, Imaging Agents, Biomarkers, stents and other drug delivery devices (covered in GlobalData’s Medical Intelligence Center).
- Nutraceuticals, dietary supplements, alternative medicines, imaging agents, radio emitter, transplants, transfusions, fillers, cosmetics, probiotics, antiseptics, antacids, mobilizing agents, veterinary drugs and drugs not seeking approval.
- Generic drugs
- Innovative drugs in Preclinical or Discovery Stage.
- Pipeline drugs sponsored by a Government or Institution.
- Drugs with a specific Drug Geography not the United States.
The probability of a drug ultimately receiving market authorization
The probability of a drug’s advancement to the next stage of clinical development
GlobalData’s Drug-Specific Likelihood of Approval (LoA) calculates the Phase Transition Success Rate (PTSR) and Likelihood of Approval (LoA) customized to individual drug. The model uses a combination of Machine Learning (ML) and a GlobalData proprietary algorithm to process data points from the Drugs, Clinical Trials, Regulatory Milestones, Company, and Financial databases.
Inclusion
Data Scope:
Drug Phase Scope:
Drug Geography Scope:
Drug Type Scope:
Entity Type Scope:
Only drugs in development by companies are included in the model.
Exclusion
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Related reports
View more reports