Malaysia – Healthcare (Pharma and Medical Devices) Market Analysis, Regulatory, Reimbursement and Competitive Landscape
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
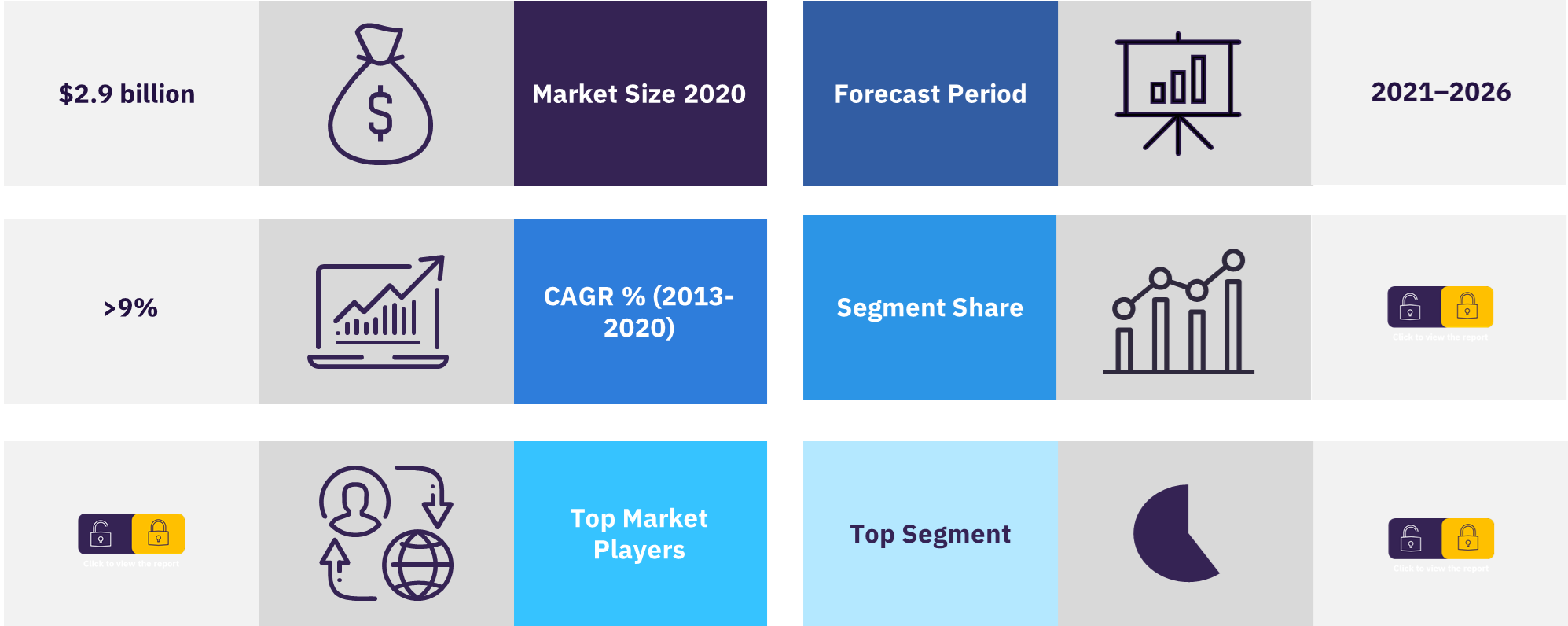
The Malaysia pharmaceutical market was valued at $2.9 billion in the year 2020. The market grew at a CAGR of more than 9% during the period 2013 to 2020. According to Malaysia’s Investment Development Authority (MIDA), as of June 2021, the pharmaceutical industry’s aggregate annual earnings before interest, taxes, depreciation, and amortization (EBITDA) is expected to grow over the next 12 to 18 months due to rise in pharmaceutical use and COVID-19-related treatments and vaccines.
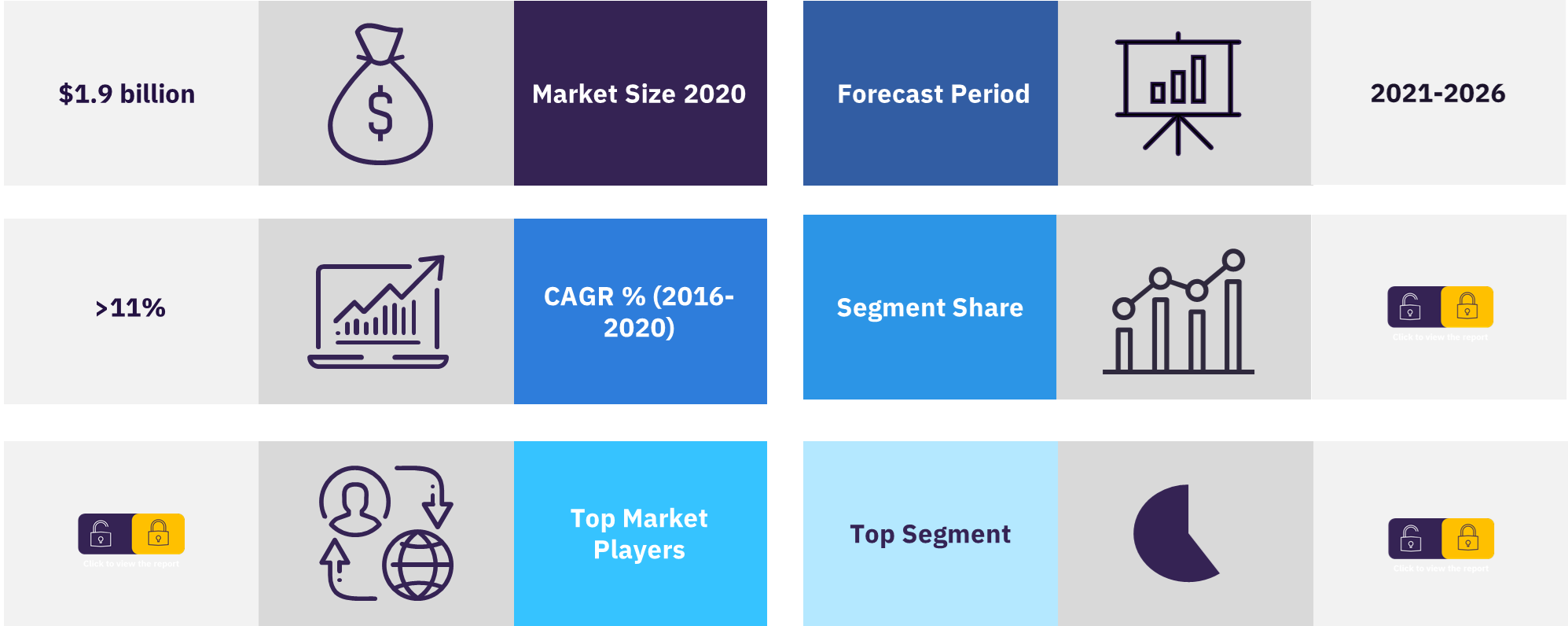
The Malaysia medical devices market was valued at $1.9 billion in the year 2020. The market grew at a CAGR of more than 11% during the period 2016 to 2020. Malaysia provides one of the strongest markets for foreign medical devices manufacturers in Southeast Asia. The medical devices industry was also identified as one of the significant growth areas under the healthcare National Key Economic Area (NKEA) 2020.
Malaysia pharmaceutical market overview
For more insights on this report, download a free sample
Malaysia medical devices market overview
For more insights on this report, download a free sample
What are the key segments in the pharmaceutical market in Malaysia?
The key segments in the pharmaceutical market in Malaysia are generics, biologics, biosimilars, and over-the-counter (OTC).
Who are the major players in the pharmaceutical market in Malaysia?
The major players in the pharmaceutical market in Malaysia are Novartis, GlaxoSmithKline (GSK), Pfizer, Y.S.P. Southeast Asia (Y.S.P. SAH), and Kotra Pharma.
GlaxoSmithKline (GSK)
It is a healthcare company that focuses on the development, manufacture, and commercialization of pharmaceuticals, vaccines, and consumer healthcare products. It offers drugs for the treatment of respiratory diseases, cancer, cardiovascular diseases, human immunodeficiency virus (HIV), infectious diseases, immuno-inflammation, and rare diseases. It is headquartered in Brentford, London. In Malaysia, it operates from Selengor.
Novartis
It is a healthcare company that focuses on the discovery, development, manufacture, and marketing of prescription and generic pharmaceutical products, as well as eye care products. It provides drugs for the treatment of cancer and other diseases. Novartis is headquartered in Basel, Switzerland. In Malaysia, it operates from Seksyen/Selangor Darul Ehsan.
Pfizer
It is a research-based global biopharmaceutical company that discovers, develops, manufactures, and commercializes drugs for various conditions, consumer healthcare products, generics, and active pharmaceutical ingredients (APIs). Pfizer is headquartered in the US. In Malaysia, it operates from Kuala Lumpur.
Malaysia pharmaceutical market, by major players
To know more about major players, download a free sample
Who are the major market players in the medical devices market in Malaysia?
The major players in the medical devices market in Malaysia are Abbott, Medtronic, GE Healthcare, Stryker Corp (Stryker), and LKL International Berhad.
Abbott
It is a diversified health product company that manufactures and markets drugs, diagnostics, branded generics, vascular products, and nutritional products. The company’s products comprise specialized medicines; medical diagnostic instruments and tests; minimally invasive surgical devices; and products for veterinary care. Its Malaysia office is located in Shah Alam, Selangor.
Medtronic
It is a medical technology company. It provides a wide range of medical devices for the treatment of heart disease, spinal conditions, neurological disorders, vascular disease, orthopedic disorders, and diabetes. Medtronic is headquartered in Dublin, Ireland. In Malaysia, Medtronic is located at Petaling Jaya, Selangor, Malaysia.
GE Healthcare
It is a business unit of General Electric Company. It manufactures and sells medical imaging devices for diagnosis, as well as other medical devices. GE Healthcare is headquartered in Chicago, Illinois, US. In Malaysia, it operates from Kuala Lumpur.
Malaysia medical devices market, by major players
To know more about major players, download a free sample
Market report scope (Malaysia Pharmaceutical Market)
| Market size 2020 | $2.9 billion |
| CAGR | >9% (2013-2020) |
| Forecast Period | 2021-2026 |
| Key segments | Generics, Biologics, Biosimilars, and Over-The-Counter (OTC) |
| Major players | Novartis, GlaxoSmithKline (GSK), Pfizer, Y.S.P. Southeast Asia (Y.S.P. SAH), and Kotra Pharma |
Market report scope (Malaysia Medical Devices Market)
| Market size 2020 | $1.9 billion |
| Forecast Period | 2021-2026 |
| CAGR | >11% (2016-2020) |
| Major players | Abbott, Medtronic, GE Healthcare, Stryker Corp (Stryker), and LKL International Berhad |
Scope
The report provides information on the healthcare, regulatory, and reimbursement landscape in Malaysia, and includes:
- An overview of the pharmaceutical and medical device markets, comprising market size, segmentation, and key drivers and barriers.
- Profiles and SWOT analyses of the major players in the pharmaceutical market: Roche, Novartis, GlaxoSmithKline (GSK), Pfizer, YSP SAH and Kotra pharma.
- Profiles and SWOT analyses of the major players in the medical device market: Abbott, Medtronic, GE Healthcare, Stryker, and LKL.
- An insightful review of the COVID-19 epidemiology, COVID-19 impact and developments in healthcare market, HealthTech landscape, reimbursement and regulatory landscape, with analysis covering details of the country’s healthcare reimbursement process, regulatory agencies and the approval processes for new drugs and medical devices.
- Detailed analysis of the country’s healthcare policy highlights, demographics, healthcare infrastructure and healthcare expenditure.
- An overview of the opportunities for and challenges to growth in the Malaysia’s healthcare market”
Reasons to Buy
- This report will enhance your decision-making capability by allowing you to:
- Develop business strategies by understanding the trends shaping and driving the Malaysia’s healthcare market
- Drive revenues by understanding the key trends, reimbursement and regulatory policies, pharmaceutical market segments and companies likely to impact the Malaysia’s healthcare market in the future
- Formulate effective sales and marketing strategies by understanding the competitive landscape and analyzing competitors’ performance
- Organize your sales and marketing efforts by identifying the market categories and segments that present the most opportunities for consolidation, investment and strategic partnership
Table of Contents
Table
Figures
Frequently asked questions
-
What was the Malaysia pharmaceutical market size in the year 2020?
The pharmaceutical market size in Malaysia was valued at $2.9 billion in the year 2020.
-
What is the Malaysia pharmaceutical market growth rate?
The pharmaceutical market in Malaysia grew at a CAGR of more than 9% during the review period.
-
What are the key segments in the Malaysia pharmaceutical market?
Generics, biologics, biosimilars, and over-the-counter (OTC) are the key segments in the Malaysian pharmaceutical market.
-
Who are the major players in the Malaysia pharmaceutical market?
Novartis, GlaxoSmithKline (GSK), Pfizer, Y.S.P. Southeast Asia (Y.S.P. SAH), and Kotra Pharma are the major players in the Malaysian pharmaceutical market.
-
What was the Malaysia medical devices market size in the year 2021?
The pharmaceutical market size in Malaysia was valued at $1.9 billion in the year 2021.
-
What is the Malaysia medical devices market growth rate?
The medical devices market in Malaysia grew at a CAGR of more than 11% during the review period.
-
Who are the major players in the Malaysia medical devices market?
Abbott, Medtronic, GE Healthcare, Stryker Corp (Stryker), and LKL International Berhad are the major players in the Malaysian medical devices market.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Pharmaceuticals reports