Multiple Myeloma (Kahler Disease) Drugs in Development by Stages, Target, MoA, RoA, Molecule Type and Key Players, 2022 Update
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Multiple Myeloma Pipeline Products Market Report Overview
Multiple myeloma is a type of cancer that is caused by malignant plasma cells that proliferate in the bone marrow and produce abnormally high amounts of a special protein. The exact cause of multiple myeloma is not clear. It mainly affects older adults. Symptoms include anemia, bleeding, nerve damage, skin lesions, bone tenderness or pain, and kidney failure. Treatment includes chemotherapy, radiation, immunosuppression, and surgery.
The Multiple Myeloma Drugs in Development market research report provides an overview of the Multiple Myeloma (Kahler Disease) pipeline landscape. The report provides comprehensive information on the therapeutics under development for Multiple Myeloma (Kahler Disease), complete with analysis by stage of development, drug target, mechanism of action (MoA), route of administration (RoA), and molecule type. The report also covers the descriptive pharmacological action of the therapeutics, its complete research and development history, and the latest news and press releases. Additionally, the report provides an overview of key players involved in therapeutic development for Multiple Myeloma (Kahler Disease) and features dormant and discontinued projects.
| Targets | Cells Expressing Tumor Necrosis Factor Receptor Superfamily Member 17, Cells Expressing ADP Ribosyl Cyclase/Cyclic ADP Ribose Hydrolase 1, CD3, Cells Expressing B Lymphocyte Antigen CD19, ADP Ribosyl Cyclase/Cyclic ADP Ribose Hydrolase 1, Cells Expressing SLAM Family Member 7, Leukocyte Surface Antigen CD47, Cells Expressing Syndecan 1, Histone Deacetylase 6, and Programmed Cell Death Protein 1 |
| Mechanisms of Action | Cytotoxic To Cells Expressing Tumor Necrosis Factor Receptor Superfamily Member 17, Cytotoxic to Cells Expressing ADP Ribosyl Cyclase/Cyclic ADP Ribose Hydrolase 1, CD3 Agonist, Cytotoxic To Cells Expressing B Lymphocyte Antigen CD19, ADP Ribosyl Cyclase/Cyclic ADP Ribose Hydrolase 1 Inhibitor, Cytotoxic To Cells Expressing SLAM Family Member 7, Leukocyte Surface Antigen CD47 Inhibitor, Cytotoxic To Cells Expressing Syndecan 1, Histone Deacetylase 6 Inhibitor, and B Cell Lymphoma 2 Inhibitor |
| Routes of Administration | Intravenous, Oral, Parenteral, Subcutaneous, Intraperitoneal, Intratumor, Intradermal, Intralesional, Intravesical, And Intravenous Drip |
| Molecule Types | Small Molecule, Gene-Modified Cell Therapy, Monoclonal Antibody, Cell Therapy, Monoclonal Antibody Conjugated, Fusion Protein, Oncolytic Virus, Subunit Vaccine, Synthetic Peptide, and Gene Therapy |
| Key Companies | Bristol-Myers Squibb Co, Novartis AG, Pfizer Inc, Johnson & Johnson, Sanofi, AbbVie Inc, Amgen Inc, Shanghai YaKe Biotechnology Co Ltd, AstraZeneca Plc, and Gilead Sciences Inc |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Multiple Myeloma Pipeline Drugs Market Segmentation by Target
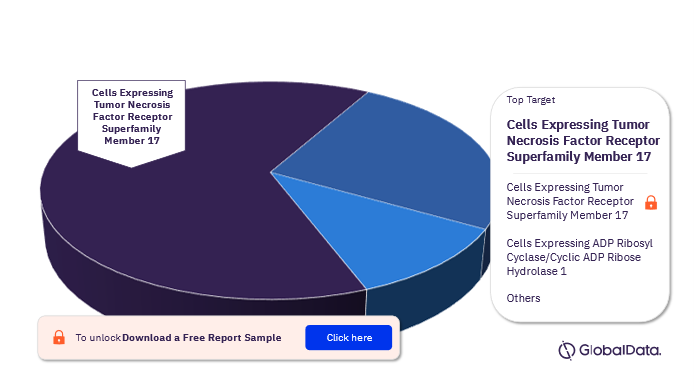
The targets of the Multiple Myeloma pipeline drugs market are Cells Expressing Tumor Necrosis Factor Receptor Superfamily Member 17, Cells Expressing ADP Ribosyl Cyclase/Cyclic ADP Ribose Hydrolase 1, CD3, and Cells Expressing B Lymphocyte Antigen CD19 among others. In 2022, the most popular targest in the Multiple Myeloma Pipeline Drugs market was Cells Expressing Tumor Necrosis Factor Receptor Superfamily Member 17.
Multiple Myeloma Pipeline Drugs Market Analysis By Targets, 2022 (%)
For more target insights in the Multiple Myeloma pipeline drugs market, download a free report sample
Multiple Myeloma Pipeline Drugs Market Segmentation by Mechanism of Action (MoA)
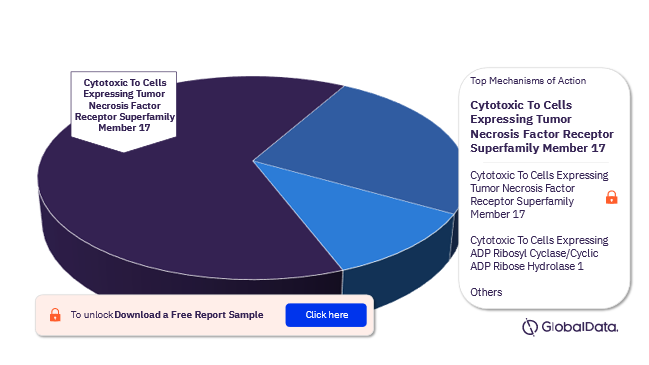
The mechanisms of action of the Multiple Myeloma pipeline drugs market are Cytotoxic To Cells Expressing Tumor Necrosis Factor Receptor Superfamily Member 17, Cytotoxic To Cells Expressing ADP Ribosyl Cyclase/Cyclic ADP Ribose Hydrolase 1, CD3 Agonist, and Cytotoxic To Cells Expressing B Lymphocyte Antigen CD19 among others. In 2022, Cytotoxic To Cells Expressing Tumor Necrosis Factor Receptor Superfamily Member 17 emerged as the leading MoA in the Multiple Myeloma pipeline drugs market.
Multiple Myeloma Pipeline Drugs Market By Mechanisms Of Action, 2022 (%)
For more MoA insights in the Multiple Myeloma Pipeline Drugs Market, download a free report sample
Multiple Myeloma Pipeline Drugs Market Segmentation by Routes of Administration (RoA)
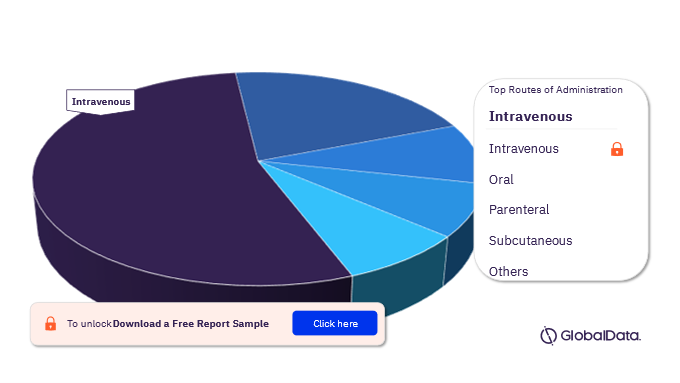
The routes of administration in the Multiple Myeloma pipeline drugs market are intravenous, oral, parenteral, subcutaneous, intraperitoneal, intratumor, intradermal, intralesional, intravesical, and intravenous drip. In 2022, intravenous RoA emerged as the largest segment.
Multiple Myeloma Pipeline Drugs Market Analysis by Routes Of Administration, 2022 (%)
For more RoA insights in the Multiple Myeloma Pipeline Drugs Market, download a free report sample
Multiple Myeloma Pipeline Drugs Market Segmentation by Molecule Types
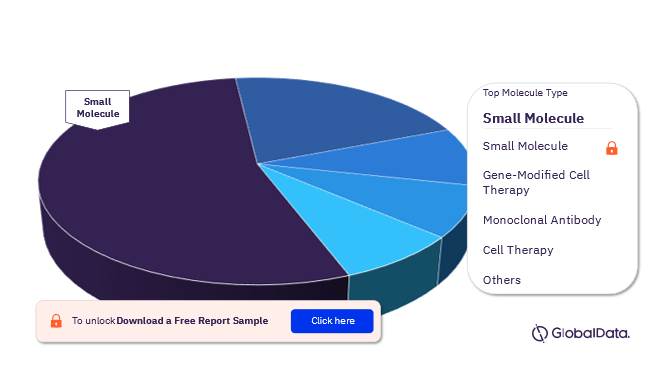
The molecule types in the Multiple Myeloma pipeline drugs market are small molecule, gene-modified cell therapy, monoclonal antibody, cell therapy, monoclonal antibody conjugated, fusion protein, oncolytic virus, subunit vaccine, synthetic peptide, and gene therapy. In 2022, small molecules were the most preferred molecule type in the Multiple Myeloma pipeline drugs market.
Multiple Myeloma Pipeline Drugs Market Analysis by Molecule Types, 2022 (%)
For more molecule type insights in the Multiple Myeloma Pipeline Drugs Market, download a free report sample
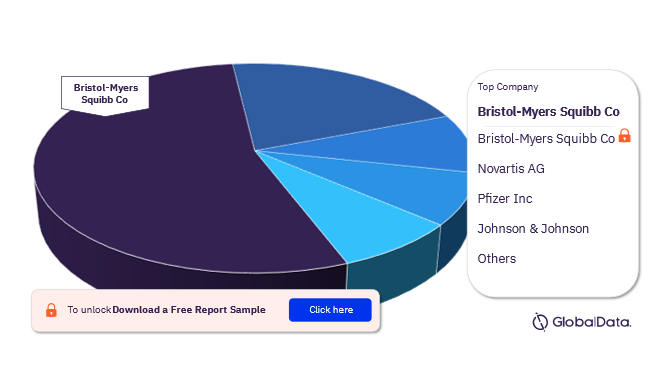
Multiple Myeloma Pipeline Products Market - Competitive Landscape
Some of the key companies in the Multiple Myeloma pipeline drugs market are Bristol-Myers Squibb Co, Novartis AG, Pfizer Inc, Johnson & Johnson, Sanofi, AbbVie Inc, Amgen Inc, Shanghai YaKe Biotechnology Co Ltd, AstraZeneca Plc, and Gilead Sciences Inc. In 2022, Bristol-Myers Squibb Co emerged as the market leader with the highest number of Multiple Myeloma pipeline drugs market.
Multiple Myeloma Pipeline Drugs Market Analysis by Key Companies, 2022 (%)
To know more about key leading companies in the Multiple Myeloma Pipeline Drugs market, download a free report sample
Key Segments Covered in this Report
Multiple Myeloma Pipeline Drugs Target Outlook (%, 2022)
- Cells Expressing Tumor Necrosis Factor Receptor Superfamily Member 17
- Cells Expressing ADP Ribosyl Cyclase/Cyclic ADP Ribose Hydrolase 1
- CD3
- Cells Expressing B Lymphocyte Antigen CD19
- Others
Multiple Myeloma Pipeline Drugs MoA Outlook (%, 2022)
- Cytotoxic To Cells Expressing Tumor Necrosis Factor Receptor Superfamily Member 17
- Cytotoxic to Cells Expressing ADP Ribosyl Cyclase/Cyclic ADP Ribose Hydrolase 1
- CD3 Agonist
- Cytotoxic To Cells Expressing B Lymphocyte Antigen CD19
- Others
Multiple Myeloma Pipeline Drugs RoA Outlook (%, 2022)
- Intravenous
- Oral
- Parenteral
- Subcutaneous
- Intraperitoneal
- Others
Multiple Myeloma Pipeline Drugs RoA Outlook (%, 2022)
- Small Molecule
- Gene-Modified Cell Therapy
- Monoclonal Antibody
- Cell Therapy
- Monoclonal Antibody Conjugated
- Others
Scope
- The pipeline guide provides a snapshot of the global therapeutic landscape of multiple myeloma.
- The pipeline guide reviews pipeline therapeutics for multiple myeloma by companies and universities/research institutes based on information derived from company and industry-specific sources.
- The pipeline guide covers pipeline products based on several stages of development ranging from pre-registration to discovery and undisclosed stages.
- The pipeline guide features descriptive drug profiles for the pipeline products which comprise, product description, descriptive licensing and collaboration details, R&D brief, MoA & other developmental activities.
- The pipeline guide reviews key companies involved in multiple myeloma therapeutics and enlists all their major and minor projects.
- The pipeline guide evaluates multiple myeloma therapeutics based on mechanism of action (MoA), drug target, route of administration (RoA), and molecule type.
- The pipeline guide encapsulates all the dormant and discontinued pipeline projects.
- The pipeline guide reviews the latest news related to pipeline therapeutics for multiple myeloma
Reasons to Buy
- Procure strategically important competitor information, analysis, and insights to formulate effective R&D strategies.
- Recognize emerging players with potentially strong product portfolios and create effective counter-strategies to gain competitive advantage.
- Find and recognize significant and varied types of therapeutics under development for multiple myeloma.
- Classify potential new clients or partners in the target demographic.
- Develop tactical initiatives by understanding the focus areas of leading companies.
- Plan mergers and acquisitions meritoriously by identifying key players and their most promising pipeline therapeutics.
- Formulate corrective measures for pipeline projects by understanding the multiple myeloma pipeline depth and focus on Indication therapeutics.
- Develop and design in-licensing and out-licensing strategies by identifying prospective partners with the most attractive projects to enhance and expand business potential and scope.
- Adjust the therapeutic portfolio by recognizing discontinued projects and understand from the know-how what drove them from the pipeline.
4SC AG
A. Menarini Industrie Farmaceutiche Riunite Srl
Abbisko Therapeutics Co Ltd
AbbVie Inc
Abcuro Inc
ABL Bio Inc
Actinium Pharmaceuticals Inc
Active Biotech AB
Adicet Bio Inc
Affimed GmbH
AKSO Biopharmaceutical Inc
Alabama Drug Discovery Alliance
Allogene Therapeutics Inc
Alloplex Biotherapeutics Inc
Alphamab Oncology
Ambrx Biopharma Inc
Amgen Inc
Anaveon AG
Antaimmu BioMed Co Ltd
Antengene Corp Ltd
Antion Biosciences SA
APIM Therapeutics AS
APO-T BV
Apollo Therapeutics LLC
Arcellx Inc
Arch Oncology Inc
Arcus Biosciences Inc
Ariz Precision Medicine Inc
Arjuna Therapeutics
Arovella Therapeutics Ltd
Ascenta Therapeutics Inc
Ascentage Pharma Group International
Aslan Pharmaceuticals Ltd
AstraZeneca Plc
Asylia Therapeutics Inc
Auransa Inc
Aurigene Discovery Technologies Ltd
Autolus Therapeutics Plc
Ayala Pharmaceuticals Inc
Bantam Pharmaceutical LLC
Bayer AG
BeiGene Ltd
Beijing Immunochina Pharmaceuticals Co Ltd
Beijing Jingda Biotechnology Co Ltd
Beijing Mabworks Biotech Co Ltd
Beijing Menlo Biotechnology Co Ltd
Beijing Sunbio Biotech Co Ltd
Bellicum Pharmaceuticals Inc
Betta Pharmaceuticals Co Ltd
BeyondSpring Inc
Biocad
BioCurate Pty Ltd
Biohaven Pharmaceutical Holding Company Ltd
Biolexis Therapeutics Inc
BioLineRx Ltd
Biomea Fusion Inc
BioTheryX Inc
BioVie Inc
Bivictrix Therapeutics PLC
Boehringer Ingelheim International GmbH
Bold Therapeutics Inc
Bristol-Myers Squibb Co
C4 Therapeutics Inc
Calithera Biosciences Inc
Cantex Pharmaceuticals Inc
Carbiogene Therapeutics Co Ltd
Caribou Biosciences Inc
CARsgen Therapeutics Ltd
Cartesian Therapeutics Inc
CASI Pharmaceuticals Inc
CDR-Life Inc
Cell Source Inc
CellCentric Ltd
Cellectar Biosciences Inc
Cellectis SA
Cellenkos Inc
Cellestia Biotech AG
Cello Therapeutics Inc
Cellular Biomedicine Group Inc
Celularity Inc
Celyad Oncology SA
Centrax International Inc
Ceptur Therapeutics Inc
Checkpoint Therapeutics Inc
Cheetah Cell Therapeutics Co Ltd
Chengdu Zenitar Biomedical Technology Co Ltd
Chia Tai Tianqing Pharmaceutical Group Co Ltd
Chimerix Inc
China Immunotech (Beijing) Biotechnology Co Ltd
Chinook Therapeutics Inc
Chong Kun Dang Pharmaceutical Corporation
Chongqing Precision Biotech Co Ltd
CiMaas BV
Cleave Therapeutics Inc
Compugen Ltd
Convalife
Corvus Pharmaceuticals Inc
CRISPR Therapeutics AG
CSPC Pharmaceutical Group Ltd
CStone Pharmaceuticals Co Ltd
Curocell Inc
Cyclacel Pharmaceuticals Inc
Cyteir Therapeutics Inc
CytoImmune Therapeutics Inc
Cytovia Holdings Inc
Daiichi Sankyo Co Ltd
Dayton Therapeutics AG
DexTech Medical AB
Diverse Biotech Inc
Dragonfly Therapeutics Inc
Dynamic Cell Therapies Inc
Eli Lilly and Co
Elpis Biopharmaceuticals Corp
EpimAb Biotherapeutics Inc
Epizyme Inc
Eternity Bioscience Inc
eTheRNA Immunotherapies NV
Eugia Pharma Specialties Ltd
Eureka Therapeutics Inc
ExCellThera Inc
Exelixis Inc
F. Hoffmann-La Roche Ltd
Fate Therapeutics Inc
Fera Pharmaceuticals LLC
Fortis Therapeutics Inc
Fusion Pharmaceuticals Inc
Gadeta BV
Galapagos NV
Galileo Research srl
Gamida Cell Ltd
Genentech USA Inc
Genmab AS
Genrix (Shanghai) Biopharmaceutical Co Ltd
Geron Corp
Gilead Sciences Inc
Ginkgo BioWorks Inc
Gliknik Inc
GlycoMimetics Inc
Glycostem Therapeutics BV
GlyTR Therapeutics Inc
GP Pharm SA
GPCR Therapeutics Inc
Gracell Biotechnologies Inc
GSK plc
GT Biopharma Inc
Guangzhou Bio-gene Technology Co Ltd
HaemaLogix Pty Ltd
Hangzhou DAC Biotech Co Ltd
Hangzhou Sumgen Biotech Co Ltd
Harbin Gloria Pharmaceuticals Co Ltd
Harbour BioMed (Guangzhou) Co Ltd
Harpoon Therapeutics Inc
Hebei Senlang Biotechnology Co Ltd
Heidelberg Pharma AG
Helix BioPharma Corp
Helocyte Biosciences Inc
Henan Genuine Biotech Co Ltd
Hinova Pharmaceuticals Co Ltd
HitGen Inc
HK inno.N Corp
Holy Stone Healthcare Co Ltd
HRAIN Biotechnology Co Ltd
Hummingbird Bioscience Pte Ltd
Hunan Siweikang Therapeutics Ltd
HuniLife Biotechnology Inc
Hutchison MediPharma Ltd
I-Mab
iCell Gene Therapeutics LLC
Iceni Pharmaceuticals Ltd
Ichnos Sciences Inc
IDAC Theranostics Inc
IDP Discovery Pharma SL
IGM Biosciences Inc
Immix BioPharma Inc
ImmuneCyte Inc
ImmuneOnco Biopharmaceuticals (Shanghai) Co Ltd
ImmuneTarget Inc
ImmunityBio Inc
Immunotech Biopharm Ltd
Immunwork Inc
Incyte Corp
Indapta Therapeutics Inc
Inflection Biosciences Ltd
Inmune Bio Inc
InnoCare Pharma Ltd
Innovent Biologics Inc
Intra-Immusg Pvt Ltd
Inventiva SA
IO Biotech Inc
Io Therapeutics Inc
Ionis Pharmaceuticals Inc
Ionova Life Science Co Ltd
Istesso Ltd
iTeos Therapeutics Inc
Iterion Therapeutics Inc
Jiangsu Carephar Pharmaceutical Co Ltd
Jiangsu Chai Tai Fenghai Pharmaceutical Co Ltd
Jiangsu Hansoh Pharmaceutical Group Co Ltd
Jiangsu Hengrui Medicine Co Ltd
Jiangsu Kanion Pharmaceutical Co Ltd
Jiangsu Zhengda Fenghai Pharmaceutical Co Ltd
Jiangxi Shanxing Biotechnology Co Ltd
JN Biosciences LLC
Johnson & Johnson
JSK Therapeutics Inc
Jubilant Therapeutics Inc
Juventas Cell Therapy Ltd
JW Pharmaceutical Corp
K36 Therapeutics Inc
KAHR medical Ltd
Kangpu Biopharmaceuticals Ltd
Kartos Therapeutics Inc
Karyopharm Therapeutics Inc
Kesios Therapeutics Ltd
KeyMed Biosciences Inc
Kirilys Therapeutics Inc
Kite Pharma Inc
Klyss Biotech Inc
Kodikaz Therapeutic Solutions Inc
Komipharm International Co Ltd
Kronos Bio Inc
KYAN Therapeutics Inc
Kymab Ltd
Laekna Therapeutics Shanghai Co Ltd
Lantern Pharma Inc
LAVA Therapeutics NV
Leadiant Biosciences Inc
Legend Biotech Corp
Les Laboratoires Servier SAS
Leukogene Therapeutics Inc
Ligand Pharmaceuticals Inc
LintonPharm Co Ltd
Lokon Pharma AB
Lotus Pharmaceutical Co Ltd
Loxo Oncology Inc
Luminary Therapeutics Inc
Luminus Biosciences Inc
Luye Pharma Group Ltd
Mablink Bioscience
Machavert Pharmaceuticals LLC
Marker Therapeutics Inc
Max Biopharma Inc
MediGene AG
MedPacto Inc
Mendus AB
Merck & Co Inc
Millennium Pharmaceuticals Inc
Miltenyi Biomedicine GmbH
MimiVax LLC
MiNK Therapeutics Inc
Modulation Therapeutics Inc
Molecular Partners AG
Molecular Templates Inc
Monte Rosa Therapeutics Inc
Morphogenesis Inc
MorphoSys AG
Multitude therapeutics Inc
Mustang Bio Inc
Mycovia Pharmaceuticals Inc
Nanexa AB
Nanjing Aimeifei Biomedical Technology Co Ltd
Nanjing Bioheng Biotech Co Ltd
Nanjing IASO Biotherapeutics Co Ltd
Nektar Therapeutics
Neomics Pharmaceuticals LLC
Neonc Technologies Inc
Nerviano Medical Sciences SRL
Newave Pharmaceutical Inc
NewBay Medical Technology Co Ltd
NGM Biopharmaceuticals Inc
Northlake International LLC
Novartis AG
Oncodesign SA
OncoFusion Therapeutics Inc
Oncolytics Biotech Inc
Oncolyze Inc
OncoMyx Therapeutics Inc
Onconova Therapeutics Inc
OncoPep Inc
Oncopeptides AB
OncoTartis Inc
OncoTherapy Science Inc
One World Cannabis Ltd
ONK Therapeutics Ltd
Ono Pharmaceutical Co Ltd
Onward Therapeutics SA
Opna Bio SA
Orgenesis Inc
ORIC Pharmaceuticals Inc
Origincell Technology Group Co Ltd
Otsuka Pharmaceutical Co Ltd
Oxcia AB
Paras Biopharmaceuticals Finland Oy
PentixaPharm GmbH
PersonGen Anke Cellular Therapeutics Co Ltd
PersonGen BioTherapeutics (Suzhou) Co Ltd
Pfizer Inc
Pharma Mar SA
Phi Pharma SA
PI Therapeutics Ltd
Pimera Inc
Pinotbio Inc
Poseida Therapeutics Inc
Precision Biosciences Inc
Pregene ShenZhen Biotechnology Co Ltd
Prelude Therapeutics Inc
Prescient Therapeutics Ltd
Promontory Therapeutics Inc
Protheragen Inc
Qilu Pharmaceutical Co Ltd
Quadriga BioSciences Inc
Qwixel Therapeutics LLC
Rapa Therapeutics LLC
RAPT Therapeutics Inc
RE-Stem Biotech Co Ltd
Recordati SpA
Regeneron Pharmaceuticals Inc
Remd Biotherapeutics Inc
SA Science Inc
Salarius Pharmaceuticals Inc
Sana Biotechnology Inc
Sanofi
Seagen Inc
SELLAS Life Sciences Group Inc
Senhwa Biosciences Inc
Shandong New Time Pharmaceutical Co Ltd
Shanghai Biomed-Union Biotechnology Co Ltd
Shanghai Bioray Laboratory Inc
Shanghai GeneChem Co Ltd
Shanghai Henlius Biotech Inc
Shanghai HyaMab Biotech Co Ltd
Shanghai Junshi Bioscience Co Ltd
Shanghai Keqi Pharmaceutical Technology Co Ltd
Shanghai Novamab Biopharmaceuticals Co Ltd
Shanghai Theorion Pharmaceutical Co Ltd
Shanghai Unicar-Therapy Bio-Medicine Technology Co Ltd
Shanghai YaKe Biotechnology Co Ltd
Shenogen Pharma Group Ltd
Shenzhen Chipscreen Biosciences Co Ltd
Shenzhen Neptunus Institute of Pharmaceutical Technology Co Ltd
Shenzhen Targetrx Inc
Shuttle Pharmaceuticals Inc
Sichuan Kelun Bio-Tech Pharmaceutical Co Ltd
Sichuan Kelun Pharmaceutical Co Ltd
SignalRx Pharmaceuticals Inc
SignPath Pharma Inc
Sorrento Therapeutics Inc
Spectrum Pharmaceuticals Inc
Sphaera Pharma Pte Ltd
SpringWorks Therapeutics Inc
Starton Therapeutics Inc
SteroTherapeutics LLC
Sumitomo Dainippon Pharma Oncology, Inc
Sunnycell Therapeutics Ltd
Sunomix Therapeutics
Sutro Biopharma Inc
Suzhou JiSheng Pharmaceutical Co Ltd
Suzhou Maximum Bio-tech Co Ltd
Synactix Pharmaceuticals Inc
SynDevRx Inc
T-Cure Bioscience Inc
T-CURX GmbH
TaiRx Inc
Takeda Pharmaceutical Co Ltd
Targazyme Inc
Targovax ASA
TC BioPharm Ltd
Telix Pharmaceuticals Ltd
Theolytics Ltd
TheraBioPharma Inc
Tianweiyuan and Biomedicine Shanghai Co Ltd
TiCARos Co Ltd
Tiziana Life Sciences Plc
Tmunity Therapeutics Inc
Transgene Biotek Ltd
Triterpenoid Therapeutics Inc
Triumvira Immunologics Inc
TSD Life Sciences
TTY Biopharm Co Ltd
Up Therapeutics Inc
UTC Therapeutics Inc
VaxCell Biotherapeutics Co Ltd
Vaxil Bio Therapeutics Ltd
Verastem Inc
Vichem Chemie Research Ltd
Vincerx Pharma Inc
Virtuoso Therapeutics Inc
Visterra Inc
Vycellix Inc
Vyriad Inc
Wellington Zhaotai Therapies Ltd
Wigen Biomedicine Technology (Shanghai) Co Ltd
WindMIL Therapeutics Inc
Wuhan Bio-Raid Biotechnology Co Ltd
Wuhan YZY Biopharma Co Ltd
XBiotech Inc
Xbrane Biopharma AB
XEME BioPharma Inc
Xencor Inc
Xi'An Yufan Biotechnology Co Ltd
Xiangxue Life Sciences
XNK Therapeutics AB
Zhengda Tianqing Pharmaceutical Group Co Ltd
Zovis Pharmaceuticals
Table of Contents
Table
Figures
Frequently asked questions
-
What are the key targets in the multiple myeloma pipeline products market?
The key targets of the multiple myeloma pipeline drugs market are Cells Expressing Tumor Necrosis Factor Receptor Superfamily Member 17, Cells Expressing ADP Ribosyl Cyclase/Cyclic ADP Ribose Hydrolase 1, CD3, Cells Expressing B Lymphocyte Antigen CD19, ADP Ribosyl Cyclase/Cyclic ADP Ribose Hydrolase 1, Cells Expressing SLAM Family Member 7, Leukocyte Surface Antigen CD47, Cells Expressing Syndecan 1, Histone Deacetylase 6, and Programmed Cell Death Protein 1.
-
What are the key mechanisms of action in the multiple myeloma pipeline products market?
The key MoA of the multiple myeloma pipeline drugs market are Cytotoxic To Cells Expressing Tumor Necrosis Factor Receptor Superfamily Member 17, Cytotoxic To Cells Expressing ADP Ribosyl Cyclase/Cyclic ADP Ribose Hydrolase 1, CD3 Agonist, Cytotoxic To Cells Expressing B Lymphocyte Antigen CD19, ADP Ribosyl Cyclase/Cyclic ADP Ribose Hydrolase 1 Inhibitor, Cytotoxic To Cells Expressing SLAM Family Member 7, Leukocyte Surface Antigen CD47 Inhibitor, Cytotoxic To Cells Expressing Syndecan 1, Histone Deacetylase 6 Inhibitor, and B Cell Lymphoma 2 Inhibitor.
-
What are the key routes of administration in the multiple myeloma pipeline products market?
The key RoA in the multiple myeloma pipeline drugs market are intravenous, oral, parenteral, subcutaneous, intraperitoneal, intratumor, intradermal, intralesional, intravesical, and intravenous drip.
-
What are the key molecule types in the multiple myeloma pipeline products market?
The key molecule types in the multiple myeloma pipeline drugs market are small molecule, gene-modified cell therapy, monoclonal antibody, cell therapy, monoclonal antibody conjugated, fusion protein, oncolytic virus, subunit vaccine, synthetic peptide, and gene therapy.
-
Which are the leading companies in the multiple myeloma pipeline products market?
Some of the key companies in the multiple myeloma pipeline drugs market are Bristol-Myers Squibb Co, Novartis AG, Pfizer Inc, Johnson & Johnson, Sanofi, AbbVie Inc, Amgen Inc, Shanghai YaKe Biotechnology Co Ltd, AstraZeneca Plc, and Gilead Sciences Inc.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Oncology reports