Muscular Dystrophy Drugs in Development by Stages, Target, MoA, RoA, Molecule Type and Key Players, 2022 Update
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Muscular Dystrophy Pipeline Drugs Market Report Overview
Muscular dystrophy is a group of diseases in which muscle fibers are unusually susceptible to damage. These damaged muscles become progressively weaker. Symptoms usually appear before age 6 and may appear as early as infancy. They may include fatigue, learning difficulties, intellectual disability, muscle weakness, and progressive difficulty walking.
The report titled ‘Muscular Dystrophy –Drugs in Development by Stages, Target, MoA, RoA, Molecule Type and Key Players, 2022 Update’, provides an overview of the Muscular Dystrophy pipeline landscape. The report also covers the descriptive pharmacological action of the therapeutics, its complete research and development history, and the latest news and press releases. Additionally, the report provides an overview of key players involved in therapeutic development for Muscular Dystrophy and features dormant and discontinued projects.
| Key Targets | Myotonin Protein Kinase, Double Homeobox Protein 4, Fukutin Related Protein, Polyadenylate Binding Protein 2, Calpain 3, Gamma Sarcoglycan,Glycogen Synthase Kinase 3 Beta, Growth/Differentiation Factor 8, Laminin Subunit Alpha 2, and RNA. |
| Key Mechanism of Actions | Double Homeobox Protein 4 Inhibitor, Myotonin Protein Kinase Inhibitor, Fukutin Related Protein Activator, Polyadenylate Binding Protein 2 Inhibitor, Calpain 3 Activator, Gamma Sarcoglycan Activator, Glycogen Synthase Kinase 3 Beta Inhibitor, Growth/Differentiation Factor 8 Inhibitor, Laminin Subunit Alpha 2 Activator, and Voltage Gated Sodium Channel Blocker. |
| Key Routes of Administration | Intravenous, Oral, Subcutaneous, Intramuscular, Parenteral, Topical, and Inhalational. |
| Key Molecule Types | Small Molecule, Gene Therapy, Antisense Oligonucleotide, Monoclonal Antibody Conjugated, Antisense RNAi Oligonucleotide, Cell Therapy, Gene-Modified Cell Therapy, Monoclonal Antibody, RNAi Gene Therapy, and Synthetic Peptide. |
| Leading Companies | Sarepta Therapeutics Inc, Atamyo Therapeutics SAS, Myogem Health Company SL, Dyne Therapeutics Inc, Avidity Biosciences Inc, Vita Therapeutics Inc, AMO Pharma Ltd, Anima Biotech Inc, Edgewise Therapeutics Inc, Myocea Inc, and Pfizer Inc |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
For more information on the muscular dystrophy pipeline drugs market overview, download free report sample
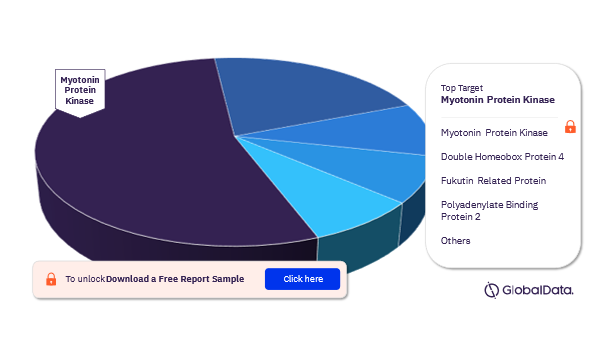
Muscular Dystrophy Pipeline Drugs Market Segmentation by Targets
The key targets of the muscular dystrophy pipeline drugs market are Mytotonin Protein Kinase, Double Homeobox Protein 4, Fukutin Related Protein, Polyadenylate Binding Protein 2, Calpain 3, Gamma Sarcoglycan, Glycogen Synthase Kinase 3 Beta, Growth/Differentiation Factor 8, Laminin Subunit Alpha 2, and RNA. In 2022, Myotonin Protein Kinase emerged as the most dominant target.
Muscular Dystrophy Pipeline Drugs Market Analysis by Targets, 2022 (%)
For additional insights on targets of the muscular dystrophy pipeline drugs market , request for a free sample report
Muscular Dystrophy Pipeline Drugs Market Segmentation by Mechanism of Actions
The key mechanism of actions of the muscular dystrophy pipeline drugs market are Double Homeobox Protein 4 Inhibitor, Myotonin Protein Kinase Inhibitor, Fukutin Related Protein Activator, Polyadenylate Binding Protein 2 Inhibitor, Calpain 3 Activator, Gamma Sarcoglycan Activator, Glycogen Synthase Kinase 3 Beta Inhibitor, Growth/Differentiation Factor 8 Inhibitor, Laminin Subunit Alpha 2 Activator, and Voltage Gated Sodium Channel Blocker. In 2022, Double Homeobox Protein 4 Inhibitor emerged as the leading mechanism of action.
Muscular Dystrophy Pipeline Drugs Market Analysis by Mechanism of Action, 2022 (%)
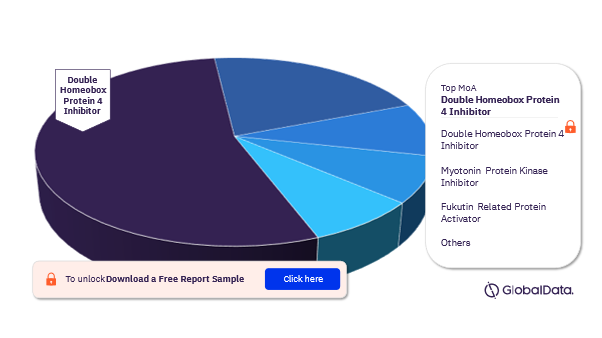 Download free sample report for more insights on the mechanism of action
Download free sample report for more insights on the mechanism of action
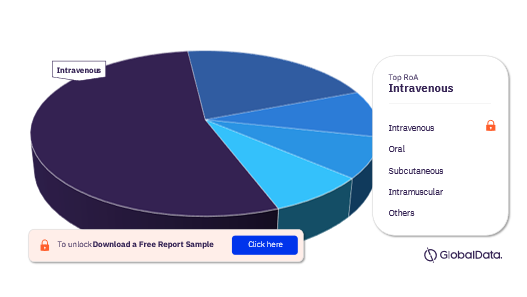
Muscular Dystrophy Pipeline Drugs Market Segmentation by Route of Administration
The key route of administration in the muscular dystrophy pipeline drugs market are intravenous, oral, subcutaneous, intramuscular, parenteral, topical, and inhalational.
Muscular Dystrophy Pipeline Drugs Market Analysis by Route of Administration, 2022 (%)
For more insights on RoA of muscular dystrophy pipeline drugs market, download free report sample
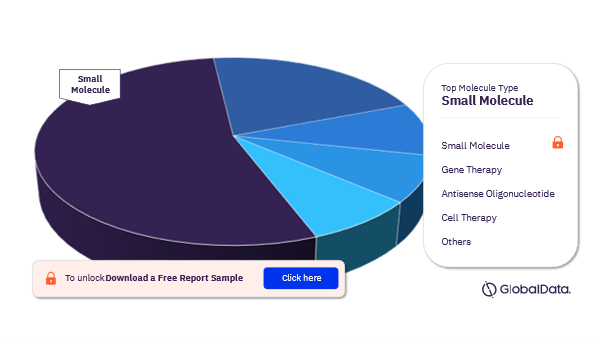
Muscular Dystrophy Pipeline Drugs Market Segmentation by Molecule Types
The main molecule types in the muscular dystrophy pipeline drugs market are Small Molecule, Gene Therapy, Antisense Oligonucleotide, Monoclonal Antibody Conjugated, Antisense RNAi Oligonucleotide, Cell Therapy, Gene-Modified Cell Therapy, Monoclonal Antibody, RNAi Gene Therapy, and Synthetic Peptide. In 2022, small molecules were the most adopted molecule type in the muscular dystrophy pipeline drugs market.
Muscular Dystrophy Pipeline Drugs Market Analysis by Molecule Types, 2022 (%)
To know more about the molecule types, download free report sample
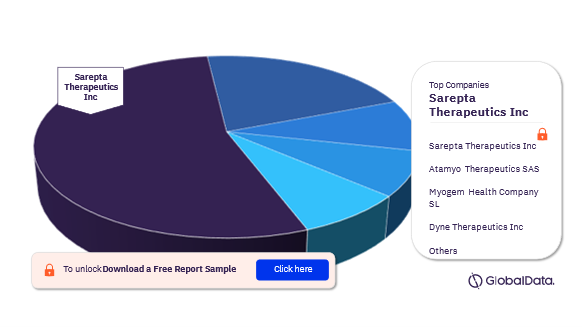
Competitive Landscape
Some of the leading companies in the muscular dystrophy pipeline drugs market are Sarepta Therapeutics Inc, Atamyo Therapeutics SAS, Myogem Health Company SL, Dyne Therapeutics Inc, Avidity Biosciences Inc, Vita Therapeutics Inc, AMO Pharma Ltd, Anima Biotech Inc, Edgewise Therapeutics Inc, Myocea Inc, and Pfizer Inc. In 2022, Sarepta Therapeutics Inc dominated the largest muscular dystrophy pipeline drugs market share with the greatest number of drugs pipeline.
Muscular Dystrophy Pipeline Drugs Market Analysis by Companies, 2022 (%)
For more information on leading companies, download a free sample report
Scope
- The pipeline guide provides a snapshot of the global therapeutic landscape of Muscular Dystrophy (Musculoskeletal Disorders).
- The pipeline guide reviews pipeline therapeutics for Muscular Dystrophy (Musculoskeletal Disorders) by companies and universities/research institutes based on information derived from company and industry-specific sources.
- The pipeline guide covers pipeline products based on several stages of development ranging from pre-registration till discovery and undisclosed stages.
- The pipeline guide features descriptive drug profiles for the pipeline products which comprise, product description, descriptive licensing and collaboration details, R&D brief, MoA & other developmental activities.
- The pipeline guide reviews key companies involved in Muscular Dystrophy (Musculoskeletal Disorders) therapeutics and enlists all their major and minor projects.
- The pipeline guide evaluates Muscular Dystrophy (Musculoskeletal Disorders) therapeutics based on mechanism of action (MoA), drug target, route of administration (RoA) and molecule type.
- The pipeline guide encapsulates all the dormant and discontinued pipeline projects.
- The pipeline guide reviews latest news related to pipeline therapeutics for Muscular Dystrophy (Musculoskeletal Disorders)
Reasons to Buy
- Procure strategically important competitor information, analysis, and insights to formulate effective R&D strategies.
- Recognize emerging players with a potentially strong product portfolio and create effective counter-strategies to gain competitive advantage.
- Find and recognize significant and varied types of therapeutics under development for Muscular Dystrophy (Musculoskeletal Disorders).
- Classify potential new clients or partners in the target demographic.
- Develop tactical initiatives by understanding the focus areas of leading companies.
- Plan mergers and acquisitions meritoriously by identifying key players and their most promising pipeline therapeutics.
- Formulate corrective measures for pipeline projects by understanding Muscular Dystrophy (Musculoskeletal Disorders) pipeline depth and focus of Indication therapeutics.
- Develop and design in-licensing and out-licensing strategies by identifying prospective partners with the most attractive projects to enhance and expand business potential and scope.
- Adjust the therapeutic portfolio by recognizing discontinued projects and understanding from the know-how what drove them from the pipeline.
Altay Therapeutics Inc
Amicus Therapeutics Inc
AMO Pharma Ltd
Anima Biotech Inc
Antisense Therapeutics Ltd
ARMGO Pharma Inc
Arrowhead Pharmaceuticals Inc
ARTHEx Biotech SL
Asklepios BioPharmaceutical Inc
Astellas Gene Therapies
Atamyo Therapeutics SAS
Avidity Biosciences Inc
Beech Tree Labs Inc
Benitec Biopharma Inc
Biophytis SA
Bioprojet SCR
CalyGene Biotechnology Inc
CANbridge Life Sciences Ltd
Casma Therapeutics Inc
Celularity Inc
Chugai Pharmaceutical Co Ltd
Constant Therapeutics LLC
CRISPR Therapeutics AG
Debiopharm International SA
Design Therapeutics Inc
DR.Noah Biotech Inc
Dyne Therapeutics Inc
Edgewise Therapeutics Inc
Elixirgen Therapeutics Inc
Entrada Therapeutics Inc
Enzerna Biosciences LLC
Epicrispr Biotechnologies Inc
EpiSwitch Rx Inc
Exodos Life Sciences Limited Partnership
Expansion Therapeutics Inc
F. Hoffmann-La Roche Ltd
Facio Therapies BV
Faze medicines
Fulcrum Therapeutics Inc
Generian Pharmaceuticals Inc
Healx Ltd
Hope Biosciences LLC
Juvena Therapeutics Inc
Keros Therapeutics Inc
LinkedUp Bioscience Inc
Locanabio Inc
Loqus23 Therapeutics Ltd
Lupin Ltd
ML Bio Solutions Inc
Modalis Therapeutics Corp
Myocea Inc
Myogem Health Company SL
NeuBase Therapeutics Inc
Nexien Biopharma Inc
Nippon Shinyaku Co Ltd
Nymirum Inc
Pasithea Therapeutics Corp
Pepgen Inc
Pfizer Inc
Phrixus Pharmaceuticals Inc
Prothelia Inc
QiXia Decode Therapeutics
Recursion Pharmaceuticals Inc
SanBio Co Ltd
Sanofi
Santhera Pharmaceuticals Holding AG
Sarcomed AB
Sarepta Therapeutics Inc
Scriptr Global Inc
SEAL Therapeutics AG
Seelos Therapeutics, Inc.
Siwa Therapeutics Inc
Syros Pharmaceuticals Inc
Triplet Therapeutics Inc
Vertex Pharmaceuticals Inc
Vita Therapeutics Inc
Xonovo Inc
Table of Contents
Table
Figures
Frequently asked questions
-
What are the key targets of the muscular dystrophy pipeline drugs market?
Myotonin Protein Kinase, Double Homeobox Protein 4, Fukutin Related Protein, Polyadenylate Binding Protein 2, Calpain 3, Gamma Sarcoglycan,Glycogen Synthase Kinase 3 Beta, Growth/Differentiation Factor 8, Laminin Subunit Alpha 2, and RNA are the key targets of the muscular dystrophy pipeline drugs market.
-
What are the key mechanism of actions of the muscular dystrophy pipeline drugs market?
The key mechanism of actions of the muscular dystrophy pipeline drugs market are Double Homeobox Protein 4 Inhibitor, Myotonin Protein Kinase Inhibitor, Fukutin Related Protein Activator, Polyadenylate Binding Protein 2 Inhibitor, Calpain 3 Activator, Gamma Sarcoglycan Activator, Glycogen Synthase Kinase 3 Beta Inhibitor, Growth/Differentiation Factor 8 Inhibitor, Laminin Subunit Alpha 2 Activator, and Voltage Gated Sodium Channel Blocker.
-
What are the key routes of administration in the muscular dystrophy pipeline drugs market?
The key route of administration in the muscular dystrophy pipeline drugs market are intravenous, oral, subcutaneous, intramuscular, parenteral, topical, and inhalational.
-
What are the key molecule types in the muscular dystrophy pipeline drugs market?
The main molecule types in the muscular dystrophy pipeline drugs market are Small Molecule, Gene Therapy, Antisense Oligonucleotide, Monoclonal Antibody Conjugated, Antisense RNAi Oligonucleotide, Cell Therapy, Gene-Modified Cell Therapy, Monoclonal Antibody, RNAi Gene Therapy, and Synthetic Peptide.
-
Which are the leading companies in the muscular dystrophy pipeline drugs market?
Some of the leading companies in the muscular dystrophy pipeline drugs market are Sarepta Therapeutics Inc, Atamyo Therapeutics SAS, Myogem Health Company SL, Dyne Therapeutics Inc, Avidity Biosciences Inc, Vita Therapeutics Inc, AMO Pharma Ltd, Anima Biotech Inc, Edgewise Therapeutics Inc, Myocea Inc, and Pfizer Inc
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Musculoskeletal Disorders reports