Neurovascular Accessory Devices Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Neurovascular Accessory Devices Pipeline Market Overview
Neurovascular accessories are devices used during neurovascular procedures to aid in the introduction of treatment devices or provide further support during the procedure. Accessories included are microguidewires, microcatheters, and distal access catheters. The Neurovascular Accessory Devices pipeline market research report provides comprehensive information about the pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials that are in progress.
| Key Segments | · Microcatheters
· Microguidewires · Distal Access Catheters |
| Key Territories | · China
· United States · Europe · Israel · Australia |
| Key Regulatory Paths | · NMPA
· 510(k) · CE · PMA · TGA |
| Leading Companies | · Achieva Medical (Shanghai) Co Ltd
· Acotec Scientific Holdings Ltd · Alcyone Therapeutics Inc · Anaconda BioMed SL · Artiria Medical SA |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Neurovascular Accessory Devices Pipeline Market Segmentation by Segments
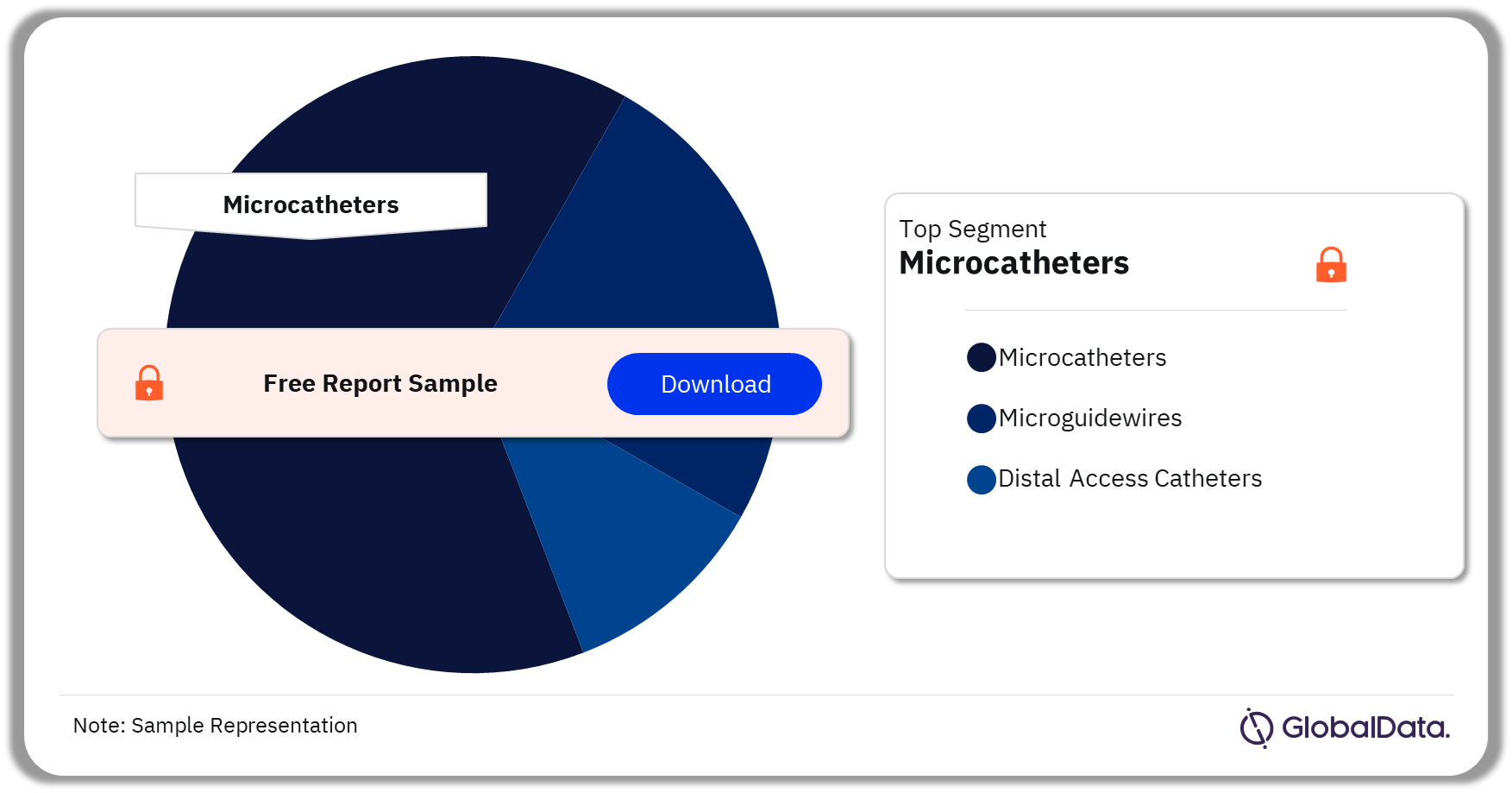
Some of the key segments in the Neurovascular Accessory Devices market are microcatheters, microguidewires, and distal access catheters. As of August 2023, Microcatheters is the leading segment in the market.
Neurovascular Accessory Devices Pipeline Market Analysis by Segments, 2023 (%)
Buy Full Report for More Segments Insights into the Neurovascular Accessory Devices Pipeline Market, Download a Free Report Sample
Neurovascular Accessory Devices Pipeline Market Segmentation by Territories
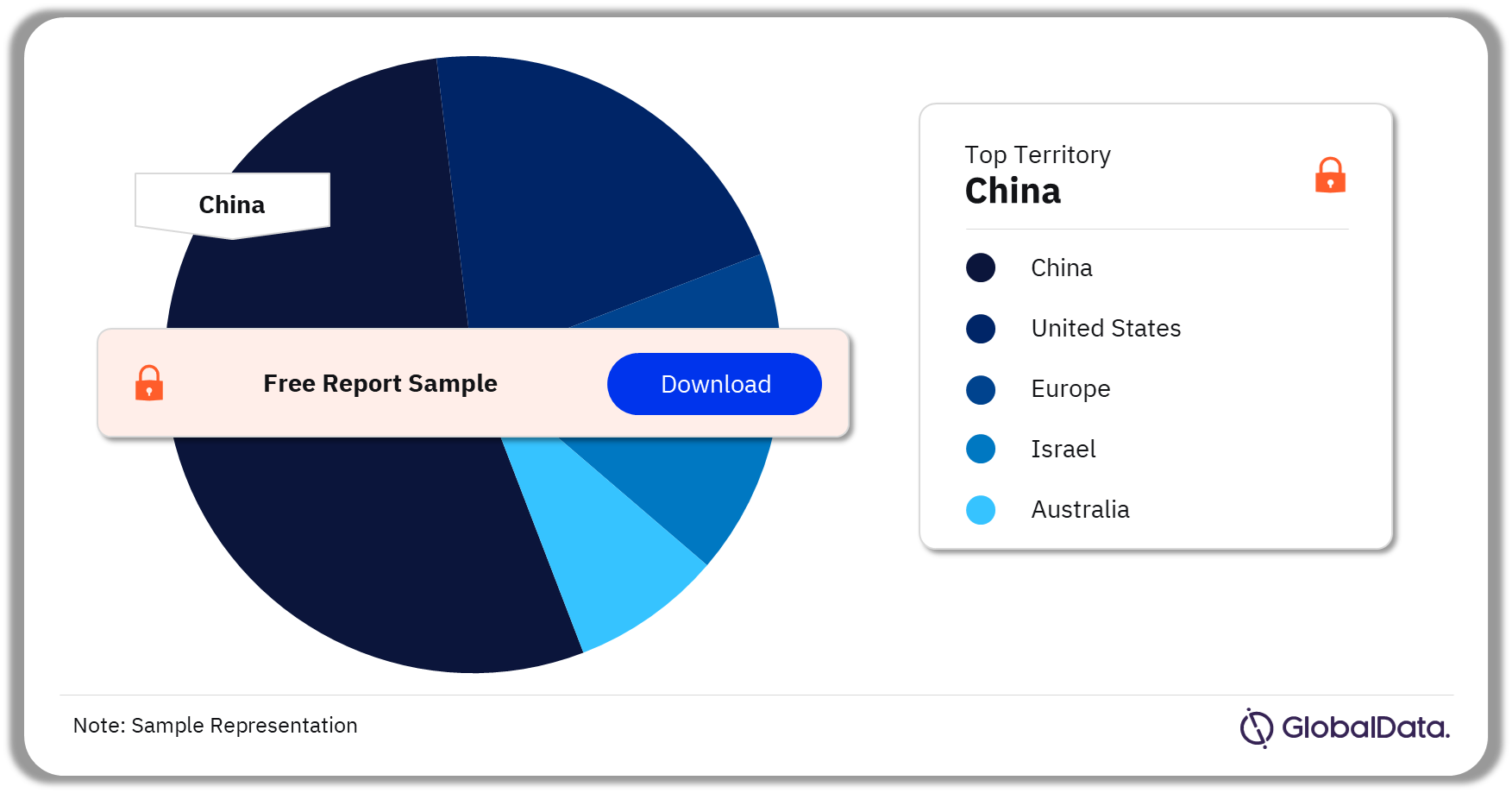
Some of the key territories in the Neurovascular Accessory Devices market are the US, China, , Europe, Australia, and Israel. As of August 2023, China accounts for the highest number of Neurovascular Accessory Devices pipeline products.
Neurovascular Accessory Devices Pipeline Market Analysis by Territories, 2023 (%)
Buy Full Report for More Territory Insights into the Neurovascular Accessory Devices Pipeline Market, Download a Free Report Sample
Neurovascular Accessory Devices Pipeline Market Segmentation by Regulatory Paths
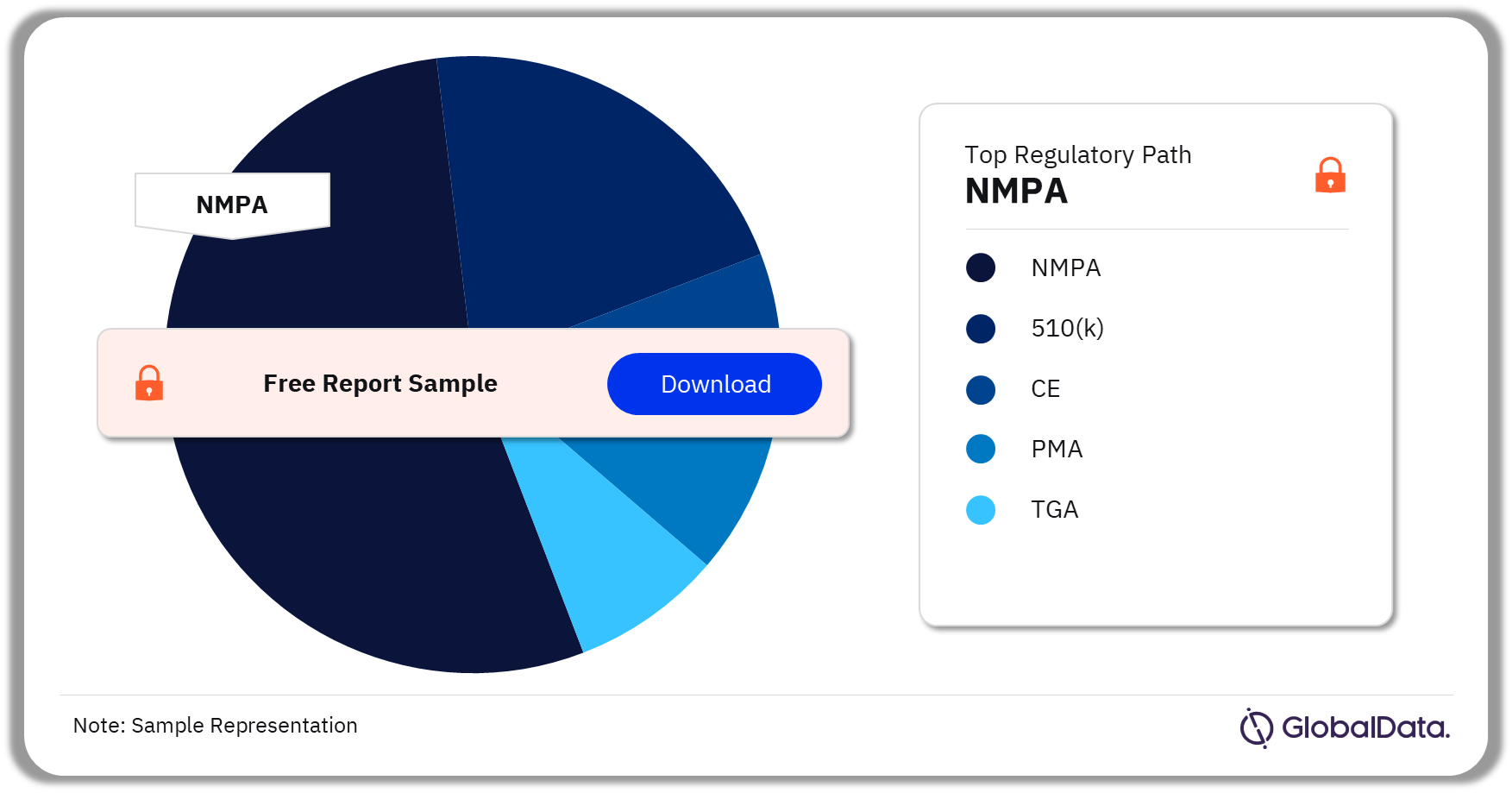
Some of the key regulatory paths in the Neurovascular Accessory Devices pipeline market are 510(k), NMPA, CE, PMA, and TGA. As of August 2023, NMPA is the most followed pathway for pipeline products.
Neurovascular Accessory Devices Pipeline Market Analysis by Regulatory Paths, 2023 (%)
Buy Full Report for More Regulatory Path Insights into the Neurovascular Accessory Devices Pipeline Market, Download a Free Report Sample
Neurovascular Accessory Devices Pipeline Market – Competitive Landscape
Some of the key companies in the Neurovascular Accessory Devices pipeline market are Achieva Medical (Shanghai) Co Ltd, Acotec Scientific Holdings Ltd, Alcyone Therapeutics Inc, Anaconda BioMed SL, and Artiria Medical SA among others.
Achieva Medical (Shanghai) Co Ltd Company Overview: Achieva Medical (Shanghai) Co Ltd (Achieva Medical), a subsidiary of Peijia Medical Ltd, is a medical device company. Its products include coils and interventional accessories. The company’s coil products include neurovascular detachable coil, a 3D design of Jasper coil that provides adherent scaffolding in aneurysm cases. Achieva Medical also carries out the development, manufacturing, and marketing the cerebral vascular interventional products. The company’s interventional products are used to treat various diseases including cerebral vascular diseases. It has a production and research and development base in Shanghai and Suzhou, China. Achieva Medical is headquartered in Shanghai, China.
Leading Neurovascular Accessory Devices Companies, 2023
Buy Full Report for More Company Insights into The Neurovascular Accessory Devices Pipeline Market, Download a Free Report Sample
Neurovascular Accessory Devices Pipeline Market Segments
Neurovascular Accessory Devices Pipeline Market Segments Outlook, (% 2023)
- Microcatheters
- Microguidewires
- Distal Access Catheters
Neurovascular Accessory Devices Pipeline Market Territories Outlook, (% 2023)
- China
- United States
- Europe
- Israel
- Australia
Neurovascular Accessory Devices Pipeline Market Regulatory Paths Outlook, (% 2023)
- NMPA
- 510(k)
- CE
- PMA
- TGA
Scope
This report provides:
- Extensive coverage of the Neurovascular Accessory Devices under development.
- Details of major pipeline products which include product description, licensing and collaboration details, and other developmental activities.
- Reviews of the major players involved in the development of Neurovascular Accessory Devices and lists of all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from Early Development to Approved/Issued stage.
- Key clinical trial data of ongoing trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with potentially strong product portfolios and create effective counter strategies to gain a competitive advantage.
- Identify and understand important and diverse types of Neurovascular Accessory Devices under development.
- Develop market-entry and market-expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date.
Acotec Scientific Holdings Ltd
Alcyone Therapeutics Inc
Anaconda BioMed SL
Artiria Medical SA
BaseCamp Vascular
Bendit Technologies Ltd
Boston University School of Medicine
Cerovations LLC
Dongguan TT Medical Inc
EchoGuide Medical, Inc. (Inactive)
Hansen Medical Inc (Inactive)
HeMo Bioengineering Ltd
Hibernia Medical
Irras AB
Medikit Co Ltd
MicroPort NeuroTech Ltd
MicroPort Scientific Corp
Navisonics Inc
Neurosyntec LLC. (Inactive)
NeuroVasc Technologies Inc
North Carolina State University
Occam Design LLC
OrbusNeich
Peijia Medical Ltd
Pennsylvania State University
Q'Apel Medical LLC
Rutgers The State University of New Jersey
Sensome SAS
Shanghai HeartCare Medical Technology Co Ltd
Shanghai Pulse Medical Technology Inc
Shenzhen Century MicroPort Medical Technology Co Ltd
Terumo Corp
Twin Star Medical Inc
University of California Los Angeles
University of California San Diego
University of Pennsylvania
University of Pittsburgh
University of Toledo
Vena Medical
Xtract Medical Inc
Zylox-Tonbridge Medical Technology Co Ltd
Table of Contents
Table
Figures
Frequently asked questions
-
Which segment has the highest number of pipeline products in the Neurovascular Accessory Devices pipeline market?
As of August 2023, Microcatheters are the leading segment in the market.
-
Which territory has the highest number of pipeline products in the Neurovascular Accessory Devices pipeline market?
As of August 2023, China has the highest number of pipeline products in the Neurovascular Accessory Devices pipeline market.
-
Which is the most followed regulatory pathway in the Neurovascular Accessory Devices pipeline market?
As of August 2023, NMPA is the most followed regulatory pathway in the Neurovascular Accessory Devices pipeline market.
-
Who are the major players operating in the Neurovascular Accessory Devices pipeline market?
Some of the key companies in the Neurovascular Accessory Devices pipeline market are Achieva Medical (Shanghai) Co Ltd, Acotec Scientific Holdings Ltd, Alcyone Therapeutics Inc, Anaconda BioMed SL, and Artiria Medical SA among others.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Neurovascular Accessory Devices reports