Ophthalmic Diagnostic Equipment – Pipeline Products by Stage of Development 31
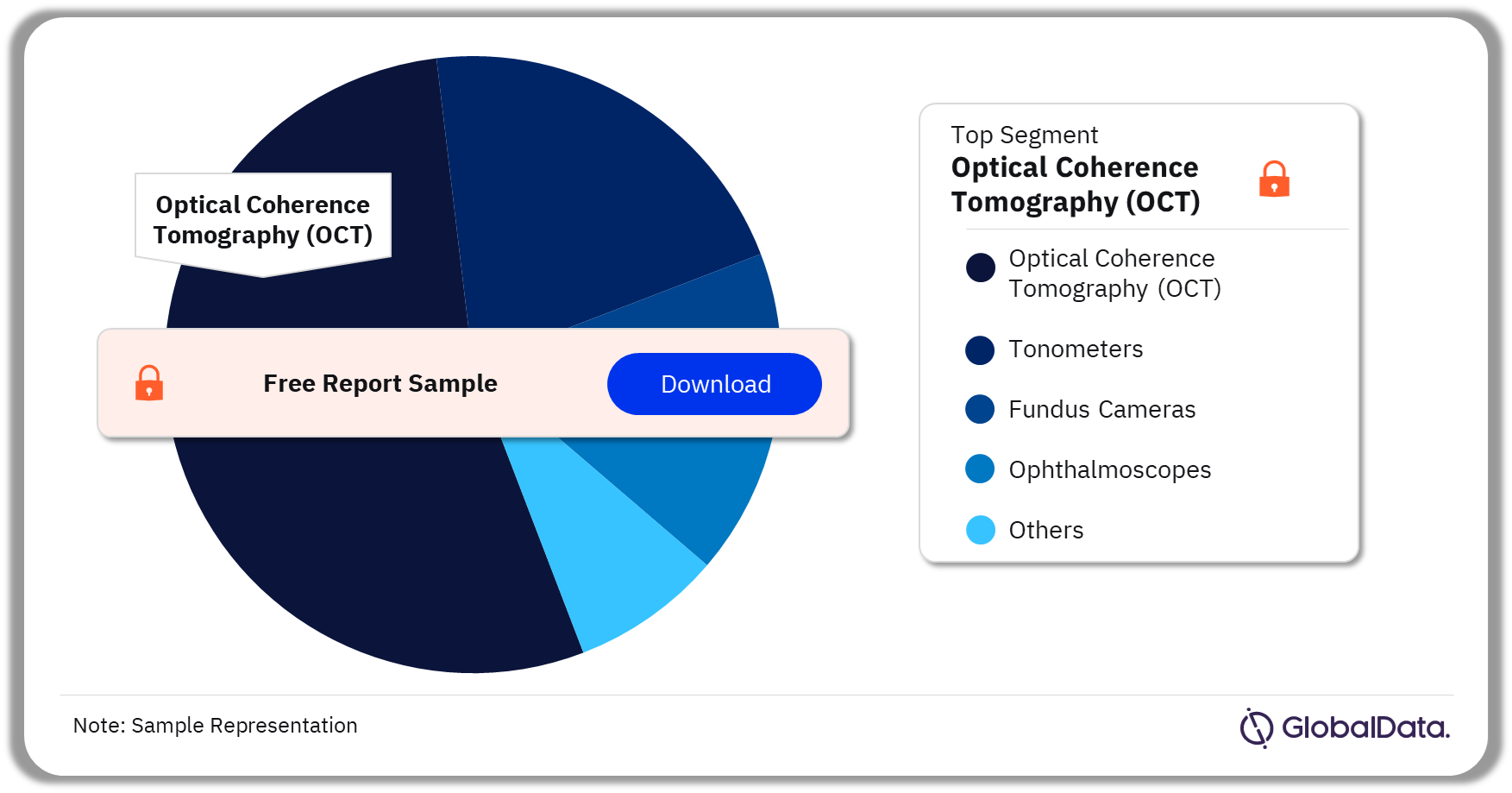
Ophthalmic Diagnostic Equipment – Pipeline Products by Segment 32
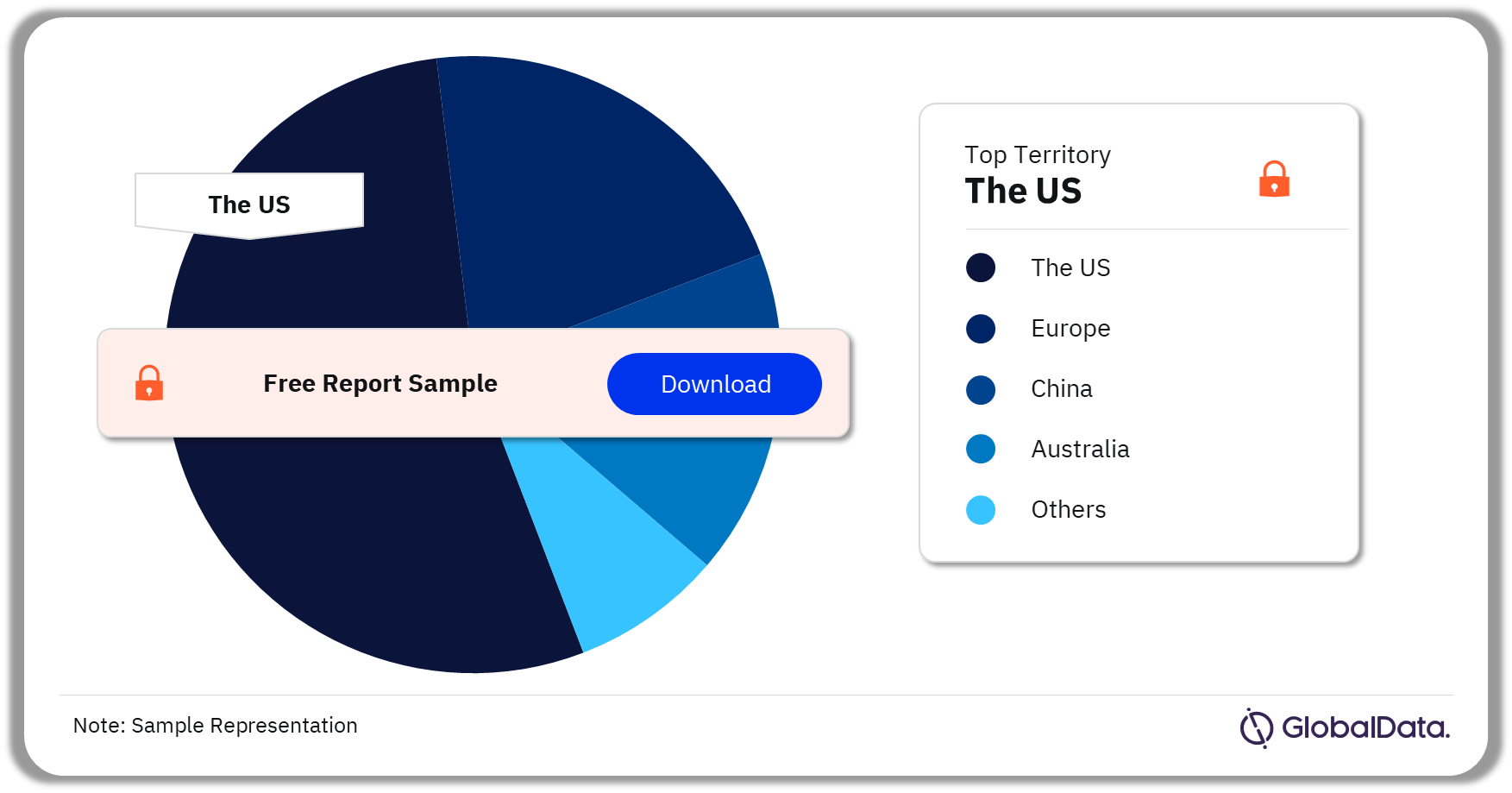
Ophthalmic Diagnostic Equipment – Pipeline Products by Territory 33
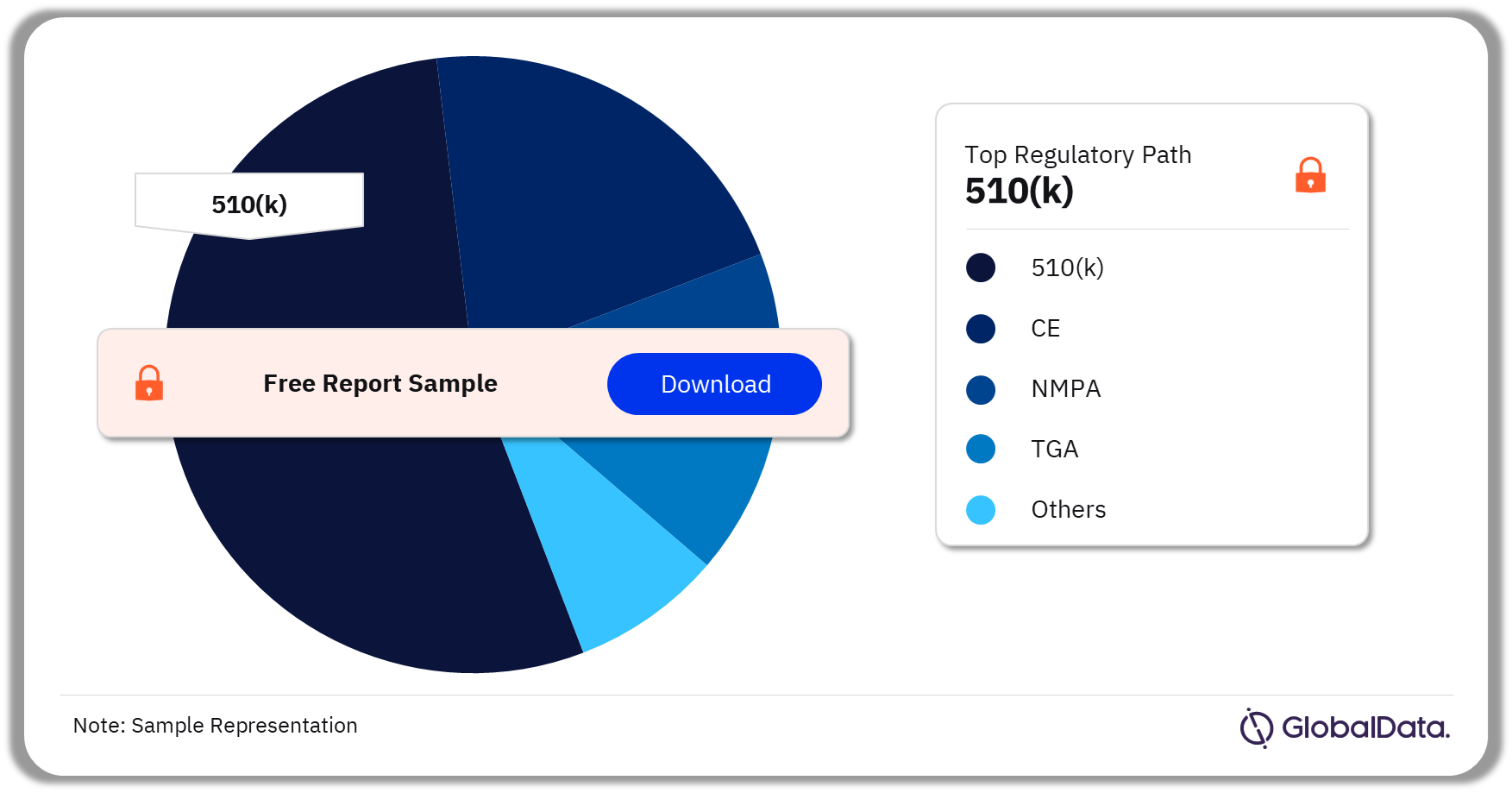
Ophthalmic Diagnostic Equipment – Pipeline Products by Regulatory Path 35
Ophthalmic Diagnostic Equipment – Pipeline Products by Estimated Approval Date 36
Ophthalmic Diagnostic Equipment – Ongoing Clinical Trials 37
Ophthalmic Diagnostic Equipment Companies – Pipeline Products by Stage of Development 38
Ophthalmic Diagnostic Equipment – Pipeline Products by Stage of Development 46
AcuMEMS Pipeline Products & Ongoing Clinical Trials Overview 53
IOP Sensor – Anterior Chamber – Product Status 53
IOP Sensor – Anterior Chamber – Product Description 53
IOP Sensor – Posterior Chamber – Product Status 54
IOP Sensor – Posterior Chamber – Product Description 54
iSense System – Product Status 54
iSense System – Product Description 55
Adom Advanced Optical Technologies Ltd Pipeline Products & Ongoing Clinical Trials Overview 56
Tear Film Imager (TFI) – COVID-19 – Product Status 56
Tear Film Imager (TFI) – COVID-19 – Product Description 56
Adom Advanced Optical Technologies Ltd – Ongoing Clinical Trials Overview 57
Tear Film Imager (TFI) – COVID-19 – Study to Compare the Efficacy of Tear Film Imager Versus Current Standard of Care for Detection of COVID-19 within the Surface of the Eye 58
Aeon Imaging, LLC Pipeline Products & Ongoing Clinical Trials Overview 59
Digital Light Ophthalmoscope (DLO) – Product Status 59
Digital Light Ophthalmoscope (DLO) – Product Description 59
Digital Light Ophthalmoscope Adaptive Optics System – Product Status 60
Digital Light Ophthalmoscope Adaptive Optics System – Product Description 60
Laser Scanning Digital Camera – Product Status 60
Laser Scanning Digital Camera – Product Description 61
Potential Vision Tester – Product Status 61
Potential Vision Tester – Product Description 61
AEYE Health Pipeline Products & Ongoing Clinical Trials Overview 62
Aurora AEYE – Product Status 62
Aurora AEYE – Product Description 62
AI Optics Inc Pipeline Products & Ongoing Clinical Trials Overview 63
Handheld Retinal Camera – Diabetic Retinopathy – Product Status 63
Handheld Retinal Camera – Diabetic Retinopathy – Product Description 63
Aireen as Pipeline Products & Ongoing Clinical Trials Overview 64
Aireen Diagnostic Scope – Age-Related Macular Degeneration – Product Status 64
Aireen Diagnostic Scope – Age-Related Macular Degeneration – Product Description 64
Aireen Diagnostic Scope – Glaucoma – Product Status 65
Aireen Diagnostic Scope – Glaucoma – Product Description 65
Alcon Inc Pipeline Products & Ongoing Clinical Trials Overview 66
All-In-One Office Diagnostic Device – Product Status 66
All-In-One Office Diagnostic Device – Product Description 66
Next-Gen Diagnostic Device – Product Status 67
Next-Gen Diagnostic Device – Product Description 67
ArcScan, Inc. Pipeline Products & Ongoing Clinical Trials Overview 68
Artemis 3 – Product Status 68
Artemis 3 – Product Description 68
Atlanta VA Medical Center Pipeline Products & Ongoing Clinical Trials Overview 69
Goggles – Retinal Disease – Product Status 69
Goggles – Retinal Disease – Product Description 69
Avedro Inc Pipeline Products & Ongoing Clinical Trials Overview 70
Brillouin Scanning Microscope – Product Status 70
Brillouin Scanning Microscope – Product Description 70
Babel Analytics LLC Pipeline Products & Ongoing Clinical Trials Overview 71
Intelligent Retinal Imaging System – Product Status 71
Intelligent Retinal Imaging System – Product Description 71
Bar-Ilan University Pipeline Products & Ongoing Clinical Trials Overview 72
Intraocular Pressure Monitoring Device – Home Use – Product Status 72
Intraocular Pressure Monitoring Device – Home Use – Product Description 72
Baylor College of Medicine Pipeline Products & Ongoing Clinical Trials Overview 73
HOCD Device – Product Status 73
HOCD Device – Product Description 73
Beaver-Visitec International Inc Pipeline Products & Ongoing Clinical Trials Overview 74
Leos – Product Status 74
Leos – Product Description 74
Miro – Product Status 75
Miro – Product Description 75
Beijing Airdoc Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 76
AI Portable Fundus Camera – Product Status 76
AI Portable Fundus Camera – Product Description 76
AI-FUNDUSCAMERA-M – Product Status 77
AI-FUNDUSCAMERA-M – Product Description 77
Ben-Gurion University of the Negev Pipeline Products & Ongoing Clinical Trials Overview 78
Diagnostic Tool – Retinal Disorders – Product Status 78
Diagnostic Tool – Retinal Disorders – Product Description 78
Beth Israel Deaconess Medical Center Pipeline Products & Ongoing Clinical Trials Overview 79
Eye Movement Marker – Huntington’s Disease – Product Status 79
Eye Movement Marker – Huntington’s Disease – Product Description 79
BioFormatix, Inc. Pipeline Products & Ongoing Clinical Trials Overview 80
VirtualEye Perimeter – Product Status 80
VirtualEye Perimeter – Product Description 80
Bioinformative Institute of India Pipeline Products & Ongoing Clinical Trials Overview 81
Ocular Surface Disease Diagnostic System – Product Status 81
Ocular Surface Disease Diagnostic System – Product Description 81
Biolight Engineering LLC Pipeline Products & Ongoing Clinical Trials Overview 82
PedCam – Product Status 82
PedCam – Product Description 82
Boston Micromachines Corporation Pipeline Products & Ongoing Clinical Trials Overview 83
Next Generation Adaptive Optics Scanning Laser Ophthalmoscope – Product Status 83
Next Generation Adaptive Optics Scanning Laser Ophthalmoscope – Product Description 83
Brien Holden Vision Institute Pipeline Products & Ongoing Clinical Trials Overview 84
Advanced Retinal Imaging System – Product Status 84
Advanced Retinal Imaging System – Product Description 84
Broadspot Imaging Corp Pipeline Products & Ongoing Clinical Trials Overview 85
Ultrawidefield Imaging Device – Product Status 85
Ultrawidefield Imaging Device – Product Description 85
C&V Tech Inc Pipeline Products & Ongoing Clinical Trials Overview 86
TONO-i Tonometer – Product Status 86
TONO-i Tonometer – Product Description 86
Cambridge University Hospitals NHS Foundation Trust Pipeline Products & Ongoing Clinical Trials Overview 87
Neocam Digital Imaging Device – Product Status 87
Neocam Digital Imaging Device – Product Description 87
Cambridge University Hospitals NHS Foundation Trust – Ongoing Clinical Trials Overview 88
Neocam Digital Imaging Device – Can the Diagnostic Accuracy of Newborn Eye Screening for Congenital Cataract be Improved With Digital Dual Light Source Imaging? The Digital Imaging vs Ophthalmoscopy (DIvO)Study 89
Canon USA Inc Pipeline Products & Ongoing Clinical Trials Overview 90
AOSLO (Adaptive Optics Scanning Laser Ophthalmoscopy) System – Product Status 90
AOSLO (Adaptive Optics Scanning Laser Ophthalmoscopy) System – Product Description 90
SPOCT HR – Product Status 91
SPOCT HR – Product Description 91
Cardiff University Pipeline Products & Ongoing Clinical Trials Overview 92
Retinal Densitometer – Product Status 92
Retinal Densitometer – Product Description 92
Carl Zeiss AG Pipeline Products & Ongoing Clinical Trials Overview 93
Multimodal Diagnostic System – Product Status 93
Multimodal Diagnostic System – Product Description 93
Case Western Reserve University Pipeline Products & Ongoing Clinical Trials Overview 94
3-D Microscopic Ultrasound System – Product Status 94
3-D Microscopic Ultrasound System – Product Description 94
Two-photon Ophthalmoscope – Product Status 95
Two-photon Ophthalmoscope – Product Description 95
CeeMax Ophthalmics Pipeline Products & Ongoing Clinical Trials Overview 96
In-Home Tonometer – Product Status 96
In-Home Tonometer – Product Description 96
Center for Biomedical Research Network in Bioengineering, Biomaterials and Nanomedicine Pipeline Products & Ongoing Clinical Trials Overview 97
Lente Sensora – Product Status 97
Lente Sensora – Product Description 97
Sensing Contact Lens – Product Status 98
Sensing Contact Lens – Product Description 98
ChromoLogic LLC Pipeline Products & Ongoing Clinical Trials Overview 99
Ocular Flare Meter – Product Status 99
Ocular Flare Meter – Product Description 99
Coland Holdings Limited Pipeline Products & Ongoing Clinical Trials Overview 100
Ophthalmic Laser Scanning Fundus Photographic Instrument – Product Status 100
Ophthalmic Laser Scanning Fundus Photographic Instrument – Product Description 100
Portable Fundus Camera – Product Status 101
Portable Fundus Camera – Product Description 101
Columbia University Pipeline Products & Ongoing Clinical Trials Overview 102
Photoacoustic Imaging System – Product Status 102
Photoacoustic Imaging System – Product Description 102
Compact Imaging Inc. Pipeline Products & Ongoing Clinical Trials Overview 103
MRO Optical Imaging Sensor – Product Status 103
MRO Optical Imaging Sensor – Product Description 103
Costruzioni Strumenti Oftalmici Cso Srl Pipeline Products & Ongoing Clinical Trials Overview 104
MD-SD-OCT Instrument – Product Status 104
MD-SD-OCT Instrument – Product Description 104
CW Optics Inc Pipeline Products & Ongoing Clinical Trials Overview 105
RetinalImager – Product Status 105
RetinalImager – Product Description 105
Dalhousie University Pipeline Products & Ongoing Clinical Trials Overview 106
Eye Imaging Device – Product Status 106
Eye Imaging Device – Product Description 106
DigitalVision, LLC Pipeline Products & Ongoing Clinical Trials Overview 107
VisionOptimizer – Product Status 107
VisionOptimizer – Product Description 107
Duke University Pipeline Products & Ongoing Clinical Trials Overview 108
HAOSLO – Product Status 108
HAOSLO – Product Description 108
HH – SDOCT – Product Status 109
HH – SDOCT – Product Description 109
Microscope Integrated OCT System – Product Status 109
Microscope Integrated OCT System – Product Description 110
OCT Scanner – Product Status 110
OCT Scanner – Product Description 110
Stereoscopic Heads-Up Display – Product Status 111
Stereoscopic Heads-Up Display – Product Description 111
Whole Eye Optical Coherence Tomography Scanner – Product Status 111
Whole Eye Optical Coherence Tomography Scanner – Product Description 112
Duke University – Ongoing Clinical Trials Overview 113
Microscope Integrated OCT System – Optimize Pediatric OCT (Optical Coherence Tomography) Imaging: a Pilot Study 114
Whole Eye Optical Coherence Tomography Scanner – Analyzing Retinal Microanatomy in Retinopathy of Prematurity to Improve Care 2 and School Age Follow on Study 115
Duke-NUS Graduate Medical School Pipeline Products & Ongoing Clinical Trials Overview 116
Pupillometer Device – Glaucoma Dx – Product Status 116
Pupillometer Device – Glaucoma Dx – Product Description 116
EarlySight SA Pipeline Products & Ongoing Clinical Trials Overview 117
Transscleral Retinal Imaging Device – Product Status 117
Transscleral Retinal Imaging Device – Product Description 117
El-Vision Ltd. Pipeline Products & Ongoing Clinical Trials Overview 118
OcuTest – Product Status 118
OcuTest – Product Description 118
Enlighten Imaging Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 119
Hyperspectral Retinal Imaging Camera – Product Status 119
Hyperspectral Retinal Imaging Camera – Product Description 119
Epvsensors, LLC Pipeline Products & Ongoing Clinical Trials Overview 120
Retinal Photometer – Product Status 120
Retinal Photometer – Product Description 120
Tactile Tonometer TT001 – Product Status 121
Tactile Tonometer TT001 – Product Description 121
Eye Care and Cure Pipeline Products & Ongoing Clinical Trials Overview 122
Diagnostic Device – Eye Alignment – Product Status 122
Diagnostic Device – Eye Alignment – Product Description 122
Eye Marker Systems Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 123
RTD 1000 – Product Status 123
RTD 1000 – Product Description 123
eyeMITRA Pipeline Products & Ongoing Clinical Trials Overview 124
eyeMITRA Device – Product Status 124
eyeMITRA Device – Product Description 124
Eyenuk Inc. Pipeline Products & Ongoing Clinical Trials Overview 125
EyeSeeAMD – Product Status 125
EyeSeeAMD – Product Description 125
EyeQue Corp Pipeline Products & Ongoing Clinical Trials Overview 126
EQ103 – Product Status 126
EQ103 – Product Description 126
EyeQue Corp – Ongoing Clinical Trials Overview 127
EQ103 – An Exploratory Study in Healthy Adult Volunteers to Evaluate the Best Corrected Visual Acuity (BCVA) Performance of a Handheld Device Compared with a Standard Eye Care Diagnostic Device in Measuring Ophthalmic Refraction 128
EQ103 – An Exploratory Study in Healthy Pediatric Volunteers to Evaluate the Performance of a Handheld Device Compared with Standard Eye Care Diagnostic Device in Measuring Ophthalmic Refraction 128
Eyoto Group Ltd Pipeline Products & Ongoing Clinical Trials Overview 129
Autorefractor – Product Status 129
Autorefractor – Product Description 129
Lensmeter – Desktop Version – Product Status 130
Lensmeter – Desktop Version – Product Description 130
Fovioptics, Inc. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 131
Retinal Blood Analyzer – Product Status 131
Retinal Blood Analyzer – Product Description 131
Gaush Meditech Ltd Pipeline Products & Ongoing Clinical Trials Overview 132
Integrated Type Slit Lamp – Product Status 132
Integrated Type Slit Lamp – Product Description 132
Gemss Co.,Ltd. Pipeline Products & Ongoing Clinical Trials Overview 133
Motorized C-arm System – Product Status 133
Motorized C-arm System – Product Description 133
George Washington University Pipeline Products & Ongoing Clinical Trials Overview 134
Amplified Bimorph Scanning Mirror System – Product Status 134
Amplified Bimorph Scanning Mirror System – Product Description 134
iEye – Product Status 135
iEye – Product Description 135
GlakoLens Biyomedikal Biyoteknoloji San ve Tic AS Pipeline Products & Ongoing Clinical Trials Overview 136
IOP Sensor – Product Status 136
IOP Sensor – Product Description 136
Hadasit Medical Research Services and Development Ltd Pipeline Products & Ongoing Clinical Trials Overview 137
Automated Visual Acuity Device – Product Status 137
Automated Visual Acuity Device – Product Description 137
Hadassah Medical Center Pipeline Products & Ongoing Clinical Trials Overview 138
Optic Neuritis Visual Test – Product Status 138
Optic Neuritis Visual Test – Product Description 138
H-Cubed Inc. Pipeline Products & Ongoing Clinical Trials Overview 139
Telemetric Microsensor – Product Status 139
Telemetric Microsensor – Product Description 139
Hedgefog Research Inc Pipeline Products & Ongoing Clinical Trials Overview 140
Ophthalmic Microendoscope – Product Status 140
Ophthalmic Microendoscope – Product Description 140
Heidelberg Engineering GmbH Pipeline Products & Ongoing Clinical Trials Overview 141
Anterion – Product Status 141
Anterion – Product Description 141
Cataract And Refractive Imaging Device – Product Status 142
Cataract And Refractive Imaging Device – Product Description 142
SPECTRALIS OCT2 Module – OCT Angiography – Product Status 142
SPECTRALIS OCT2 Module – OCT Angiography – Product Description 143
Heidelberg Engineering GmbH – Ongoing Clinical Trials Overview 144
Anterion – Comparison of Three Swept-source Optical Coherence Biometry Devices in Long and Short Eyes 145
Heriot-Watt University Pipeline Products & Ongoing Clinical Trials Overview 146
Non-Invasive Cataract Diagnostic Tool – Product Status 146
Non-Invasive Cataract Diagnostic Tool – Product Description 146
Hong Kong Polytechnic University Pipeline Products & Ongoing Clinical Trials Overview 147
Diabetic Retinopathy Detection And Monitoring System – Product Status 147
Diabetic Retinopathy Detection And Monitoring System – Product Description 147
IDx LLC Pipeline Products & Ongoing Clinical Trials Overview 148
IDx – Glaucoma – Product Status 148
IDx – Glaucoma – Product Description 148
I-MED Pharma Inc Pipeline Products & Ongoing Clinical Trials Overview 149
i-Pen Second Generation – Product Status 149
i-Pen Second Generation – Product Description 149
Imperial College London Pipeline Products & Ongoing Clinical Trials Overview 150
Intraocular Wireless Pressure Sensing Implant – Product Status 150
Intraocular Wireless Pressure Sensing Implant – Product Description 150
Implandata Ophthalmic Products GmbH Pipeline Products & Ongoing Clinical Trials Overview 151
ARGOS-EO – Product Status 151
ARGOS-EO – Product Description 151
EYEMATE-IO – Product Status 152
EYEMATE-IO – Product Description 152
EYEMATE-SC – Product Status 152
EYEMATE-SC – Product Description 153
Indian Institute of Technology Delhi Pipeline Products & Ongoing Clinical Trials Overview 154
Fundus Imaging System – Diabetic Retinopathy – Product Status 154
Fundus Imaging System – Diabetic Retinopathy – Product Description 154
Intelon Optics, Inc Pipeline Products & Ongoing Clinical Trials Overview 155
Brillouin Optical Scanner – Product Status 155
Brillouin Optical Scanner – Product Description 155
i-Optics BV Pipeline Products & Ongoing Clinical Trials Overview 156
1KHz Retina Tracker – Product Status 156
1KHz Retina Tracker – Product Description 156
Superresolution Image Enhancement – Product Status 157
Superresolution Image Enhancement – Product Description 157
iSonic Medical Pipeline Products & Ongoing Clinical Trials Overview 158
Telemedical Homecare Tonometer – Product Status 158
Telemedical Homecare Tonometer – Product Description 158
Johns Hopkins University Pipeline Products & Ongoing Clinical Trials Overview 159
Fundus Imaging System – Product Status 159
Fundus Imaging System – Product Description 159
Kaleyedos Pipeline Products & Ongoing Clinical Trials Overview 160
Wireless Retinal Imaging Device – Product Status 160
Wireless Retinal Imaging Device – Product Description 160
Kowa Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 161
VX-10a – Fundus Autofluorescence – Product Status 161
VX-10a – Fundus Autofluorescence – Product Description 162
Kubota Vision Inc Pipeline Products & Ongoing Clinical Trials Overview 163
eyeMO – Product Status 163
eyeMO – Product Description 163
Kubota Vision Inc – Ongoing Clinical Trials Overview 164
eyeMO – A Prospective Study of Patient Based Ophthalmology Suite (PBOS), In-home Optical Coherence Tomography Device in Patients of Japan 165
LaunchPoint Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 166
Intraocular Pressure Sensor – Product Status 166
Intraocular Pressure Sensor – Product Description 166
Leica Microsystems GmbH Pipeline Products & Ongoing Clinical Trials Overview 167
DI-SDOCT Imaging System – Product Status 167
DI-SDOCT Imaging System – Product Description 167
SDOCT Scanner – Product Status 168
SDOCT Scanner – Product Description 168
LighTopTech Corp Pipeline Products & Ongoing Clinical Trials Overview 169
Gabor-Domain Optical Coherence Microscopy Imaging System – Product Status 169
Gabor-Domain Optical Coherence Microscopy Imaging System – Product Description 169
Loughborough University Pipeline Products & Ongoing Clinical Trials Overview 170
Diagnostic Device – Keratoconus – Product Status 170
Diagnostic Device – Keratoconus – Product Description 170
LucyDx LLC Pipeline Products & Ongoing Clinical Trials Overview 171
Diagnostic Device – Diabetic Retinopathy – Product Status 171
Diagnostic Device – Diabetic Retinopathy – Product Description 171
Lumedica Inc Pipeline Products & Ongoing Clinical Trials Overview 172
OCTA System – Product Status 172
OCTA System – Product Description 172
Optical Coherence Tomography System – Product Status 173
Optical Coherence Tomography System – Product Description 173
Lumetrics Inc Pipeline Products & Ongoing Clinical Trials Overview 174
Fiber-Optic Eye Measurement System – Product Status 174
Fiber-Optic Eye Measurement System – Product Description 174
Fundus Camera – Product Status 175
Fundus Camera – Product Description 175
Lumisoft Technologies Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 176
Digital Gonio Camera – Product Status 176
Digital Gonio Camera – Product Description 176
Digital Slit Lamp – Product Status 177
Digital Slit Lamp – Product Description 177
Lygent Inc Pipeline Products & Ongoing Clinical Trials Overview 178
iStrab – Product Status 178
iStrab – Product Description 178
MacCure Pipeline Products & Ongoing Clinical Trials Overview 179
Opthalmic Diagnostic Device – Macular Degeneration – Product Status 179
Opthalmic Diagnostic Device – Macular Degeneration – Product Description 179
Massachusetts Eye and Ear Infirmary Pipeline Products & Ongoing Clinical Trials Overview 180
Portable Mydriatic Cameras – Product Status 180
Portable Mydriatic Cameras – Product Description 180
Retina Camera – Product Status 181
Retina Camera – Product Description 181
Medical University of South Carolina Pipeline Products & Ongoing Clinical Trials Overview 182
Retinal Disease Evaluation Device – Product Status 182
Retinal Disease Evaluation Device – Product Description 182
Michigan State University Pipeline Products & Ongoing Clinical Trials Overview 183
Intraocular Pressure Sensor – Product Status 183
Intraocular Pressure Sensor – Product Description 183
MicroPort Scientific Corp Pipeline Products & Ongoing Clinical Trials Overview 184
New Generation OCT – Product Status 184
New Generation OCT – Product Description 184
Moorfields Eye Hospital NHS Foundation Trust Pipeline Products & Ongoing Clinical Trials Overview 185
Binocular Optical Coherence Tomography – Product Status 185
Binocular Optical Coherence Tomography – Product Description 185
Nagasaki University Pipeline Products & Ongoing Clinical Trials Overview 186
Corneal Trans-Epithelial Electrical Resistance Measurement Device – Product Status 186
Corneal Trans-Epithelial Electrical Resistance Measurement Device – Product Description 186
National Taiwan University Hospital Pipeline Products & Ongoing Clinical Trials Overview 187
Pupillometer – Product Status 187
Pupillometer – Product Description 187
National University Hospital Pipeline Products & Ongoing Clinical Trials Overview 188
SUPRA Perimetry Device – Product Status 188
SUPRA Perimetry Device – Product Description 188
National University of Singapore Pipeline Products & Ongoing Clinical Trials Overview 189
Victaz Device – Product Status 189
Victaz Device – Product Description 189
Neuro Kinetics Inc Pipeline Products & Ongoing Clinical Trials Overview 190
I-Portal Retinopathy – Product Status 190
I-Portal Retinopathy – Product Description 190
NeuroFieldz Inc Pipeline Products & Ongoing Clinical Trials Overview 191
NeuroDotVR – Product Status 191
NeuroDotVR – Product Description 191
Neurolens Inc Pipeline Products & Ongoing Clinical Trials Overview 192
nMD2 – Product Status 192
nMD2 – Product Description 192
Neuroptics Inc. Pipeline Products & Ongoing Clinical Trials Overview 193
A-100 Device – Product Status 193
A-100 Device – Product Description 193
Next Dimension Ltd. Pipeline Products & Ongoing Clinical Trials Overview 194
Retinal Spectral Imaging System – Product Status 194
Retinal Spectral Imaging System – Product Description 194
nGoggle Inc Pipeline Products & Ongoing Clinical Trials Overview 195
NGoggle – Product Status 195
NGoggle – Product Description 195
nGoggle Inc – Ongoing Clinical Trials Overview 196
NGoggle – Assessment of Visual Function with a Portable Brain-computer Interface 197
Nippon Telegraph and Telephone Corp Pipeline Products & Ongoing Clinical Trials Overview 198
High – Speed Wavelength Swept Light – Based OCT Device – Product Status 198
High – Speed Wavelength Swept Light – Based OCT Device – Product Description 198
Notal Vision Inc Pipeline Products & Ongoing Clinical Trials Overview 199
Notal Home OCT System – Product Status 199
Notal Home OCT System – Product Description 199
Notal Vision Inc – Ongoing Clinical Trials Overview 200
Notal Home OCT System – Evaluation of Repeated, In-Clinic, Self-Imaging by DME Patients Using the Notal Vision 201
Notal Home OCT System – Home OCT Fluid Visualization Agreement Study at Multiple Retinal Clinics with Placement of One Home OCT Device in the Home of Each NV-AMD Subject in the US for 5 Weeks 201
Notal Home OCT System – Home OCT Guided Management Study of Subjects Diagnosed With Neovascular-AMD 201
Notal Home OCT System – Home OCT-Guided Treatment Versus Treat and Extend for the Management of Neovascular AMD 202
Nova Southeastern University Pipeline Products & Ongoing Clinical Trials Overview 203
Visual Field Evaluation Equipment – Product Status 203
Visual Field Evaluation Equipment – Product Description 203
NovaSight Pipeline Products & Ongoing Clinical Trials Overview 204
EyeSwift Pro – Product Status 204
EyeSwift Pro – Product Description 204
NovaSight – Ongoing Clinical Trials Overview 205
EyeSwift Pro – Prospective Clinical Trials to Assess Performance and Safety of EyeSwift Pro for Assessment of Ocular and Visual Functions 206
Objective Acuity Ltd Pipeline Products & Ongoing Clinical Trials Overview 207
Objective Acuity System – Product Status 207
Objective Acuity System – Product Description 207
Occuity Ltd Pipeline Products & Ongoing Clinical Trials Overview 208
AX1 Axiometer – Product Status 208
AX1 Axiometer – Product Description 208
Occuity PM1 Pachymeter – Product Status 209
Occuity PM1 Pachymeter – Product Description 209
OcuSciences, Inc. Pipeline Products & Ongoing Clinical Trials Overview 210
OcuMet Beacon – Product Status 210
OcuMet Beacon – Product Description 210
Ocutronics, LLC Pipeline Products & Ongoing Clinical Trials Overview 211
Ocutronics OC-100 Retinal Camera – Product Status 211
Ocutronics OC-100 Retinal Camera – Product Description 211
oDocs Eye Care Ltd Pipeline Products & Ongoing Clinical Trials Overview 212
visoClip – Product Status 212
visoClip – Product Description 212
visoScope – Product Status 213
visoScope – Product Description 213
Ohio State University Pipeline Products & Ongoing Clinical Trials Overview 214
Interferometric Imaging System – Product Status 214
Interferometric Imaging System – Product Description 214
Oivi AS Pipeline Products & Ongoing Clinical Trials Overview 215
Oivi Fundus Camera – Product Status 215
Oivi Fundus Camera – Product Description 215
OKO Icare Solutions Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 216
DigiON Glaucoma Screening Device – Product Status 216
DigiON Glaucoma Screening Device – Product Description 216
Ophthalmic Sciences Ltd Pipeline Products & Ongoing Clinical Trials Overview 217
IOPerfect – Product Status 217
IOPerfect – Product Description 217
IOPerfect Plus – Product Status 218
IOPerfect Plus – Product Description 218
Opticent Inc Pipeline Products & Ongoing Clinical Trials Overview 219
Aurora X3 – Product Status 219
Aurora X3 – Product Description 219
SS-vis-OCT – Product Status 220
SS-vis-OCT – Product Description 220
Optomed Oy Pipeline Products & Ongoing Clinical Trials Overview 221
Aurora IQ – Diabetic Retinopathy – Product Status 221
Aurora IQ – Diabetic Retinopathy – Product Description 222
OptoQuest Inc Pipeline Products & Ongoing Clinical Trials Overview 223
Vectralis – Product Status 223
Vectralis – Product Description 223
Optos Plc Pipeline Products & Ongoing Clinical Trials Overview 224
200TX Replacement Device – Product Status 224
200TX Replacement Device – Product Description 225
CHARLOTTE – Product Status 225
CHARLOTTE – Product Description 225
Early Detection Device – Eye Disease – Product Status 226
Early Detection Device – Eye Disease – Product Description 226
Falcon I – Product Status 226
Falcon I – Product Description 227
Falcon II – Product Status 227
Falcon II – Product Description 227
INDIANAPOLIS – Product Status 228
INDIANAPOLIS – Product Description 228
Lotte – Product Status 228
Lotte – Product Description 229
Optos Plc – Ongoing Clinical Trials Overview 230
200TX Replacement Device – A Pilot Study to Assess the Feasibility and Safety of MB-102 in Ocular Angiography as Compared to Fluorescein Sodium 231
OptoVibronex LLC Pipeline Products & Ongoing Clinical Trials Overview 232
OptoScope 2.0 – Product Status 232
OptoScope 2.0 – Product Description 232
Optovue Inc Pipeline Products & Ongoing Clinical Trials Overview 233
Intelligent OCT Retinal Disease Screening System – Product Status 233
Intelligent OCT Retinal Disease Screening System – Product Description 233
Oregon Health & Science University Pipeline Products & Ongoing Clinical Trials Overview 234
OCTA Test – Product Status 234
OCTA Test – Product Description 234
Oxford University Innovation Ltd Pipeline Products & Ongoing Clinical Trials Overview 235
Adaptive Optics Scanning Laser Ophthalmoscope – Product Status 235
Adaptive Optics Scanning Laser Ophthalmoscope – Product Description 235
PAIR Technologies LLC Pipeline Products & Ongoing Clinical Trials Overview 236
Cataract Diagnostic Device – Product Status 236
Cataract Diagnostic Device – Product Description 236
Paradigm Medical Industries, Inc. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 237
Paramax – Product Status 237
Paramax – Product Description 237
PeriVision Pipeline Products & Ongoing Clinical Trials Overview 238
Virtual Eye Test – Product Status 238
Virtual Eye Test – Product Description 238
Physical Sciences Inc Pipeline Products & Ongoing Clinical Trials Overview 239
AO-OCT System – Product Status 239
AO-OCT System – Product Description 239
Multimodal Adaptive Optics Retinal Imager (MAORI) – Product Status 240
Multimodal Adaptive Optics Retinal Imager (MAORI) – Product Description 240
PlenOptica Inc Pipeline Products & Ongoing Clinical Trials Overview 241
QuickSee Plus – Product Status 241
QuickSee Plus – Product Description 241
Predictek Inc Pipeline Products & Ongoing Clinical Trials Overview 242
IRis – Product Status 242
IRis – Product Description 242
Profundus AB Pipeline Products & Ongoing Clinical Trials Overview 243
Guide Star System – Product Status 243
Guide Star System – Product Description 243
REBIScan Inc Pipeline Products & Ongoing Clinical Trials Overview 244
Retinal Birefringence Imager – Product Status 244
Retinal Birefringence Imager – Product Description 244
Traq – Product Status 245
Traq – Product Description 245
REBIScan Inc – Ongoing Clinical Trials Overview 246
Traq – Head and Intraocular Trauma Tool for the Identification of Mild Traumatic Brain Injury 247
Regensight Srl Pipeline Products & Ongoing Clinical Trials Overview 248
TheraCXL – Keratoconus – Product Status 248
TheraCXL – Keratoconus – Product Description 248
Theravision – Low-Grade Myopia – Product Status 249
Theravision – Low-Grade Myopia – Product Description 249
Theravision – Presbyopia – Product Status 249
Theravision – Presbyopia – Product Description 250
Reichert Inc Pipeline Products & Ongoing Clinical Trials Overview 251
Tono-Vera Tonometer – Product Status 251
Tono-Vera Tonometer – Product Description 251
Reichert Inc – Ongoing Clinical Trials Overview 252
Tono-Vera Tonometer – Comparison of Intraocular Pressure Measurement with the Novel TonoVera Device with Other Commonly Used Devices 253
Rensselaer Polytechnic Institute Pipeline Products & Ongoing Clinical Trials Overview 254
Scheimpflug System – Product Status 254
Scheimpflug System – Product Description 254
RetinalGenix, Inc. Pipeline Products & Ongoing Clinical Trials Overview 255
RetinalGeniX Imaging System – Product Status 255
RetinalGeniX Imaging System – Product Description 255
Retinostics Pipeline Products & Ongoing Clinical Trials Overview 256
Ultra-high Resolution Scanning Laser Ophthalmoscope – Product Status 256
Ultra-high Resolution Scanning Laser Ophthalmoscope – Product Description 256
RetinScan Pipeline Products & Ongoing Clinical Trials Overview 257
Diabetics Retinopathy Digital Screening Device – Product Status 257
Diabetics Retinopathy Digital Screening Device – Product Description 257
RetInSight GmbH Pipeline Products & Ongoing Clinical Trials Overview 258
OCT-Based Screening Algorithm – Geographic Atrophy – Product Status 258
OCT-Based Screening Algorithm – Geographic Atrophy – Product Description 258
Retispec Inc Pipeline Products & Ongoing Clinical Trials Overview 259
Retispec – Product Status 259
Retispec – Product Description 259
Reyedar BV Pipeline Products & Ongoing Clinical Trials Overview 260
SONDA – Eye Diseases – Product Status 260
SONDA – Eye Diseases – Product Description 260
Rice University Pipeline Products & Ongoing Clinical Trials Overview 261
Diagnostic Device – Dry Eye – Product Status 261
Diagnostic Device – Dry Eye – Product Description 261
Rowiak GmbH Pipeline Products & Ongoing Clinical Trials Overview 262
Pencil OCT – Product Status 262
Pencil OCT – Product Description 262
Rutgers The State University of New Jersey Pipeline Products & Ongoing Clinical Trials Overview 263
Tonometer – Product Status 263
Tonomet
![]()