Peripheral Vascular Stents Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Peripheral Vascular Stents Pipeline Products Market Report Overview
A peripheral vascular stent is an expandable perforated tube that is inserted into a peripheral vessel to prevent blood flow constriction. The Peripheral Vascular Stents pipeline market research report provides comprehensive information about the Peripheral Vascular Stents pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
Peripheral Vascular Stents Pipeline Products Market Segments
The key segments in the Peripheral Vascular Stents Pipeline Products market are Bioabsorbable Stents (BAS), Drug Eluting Stents (DES), Iliac Artery Stents, Tracheobronchial Stent, and Bare Metal Stents (BMS). As of February 2023, Bioabsorbable Stents (BAS) dominated the market.
Peripheral Vascular Stents Pipeline Products Market Analysis by Segments, 2023 (%)
For more segments insights into the Peripheral Vascular Stents pipeline products market, download a free report sample
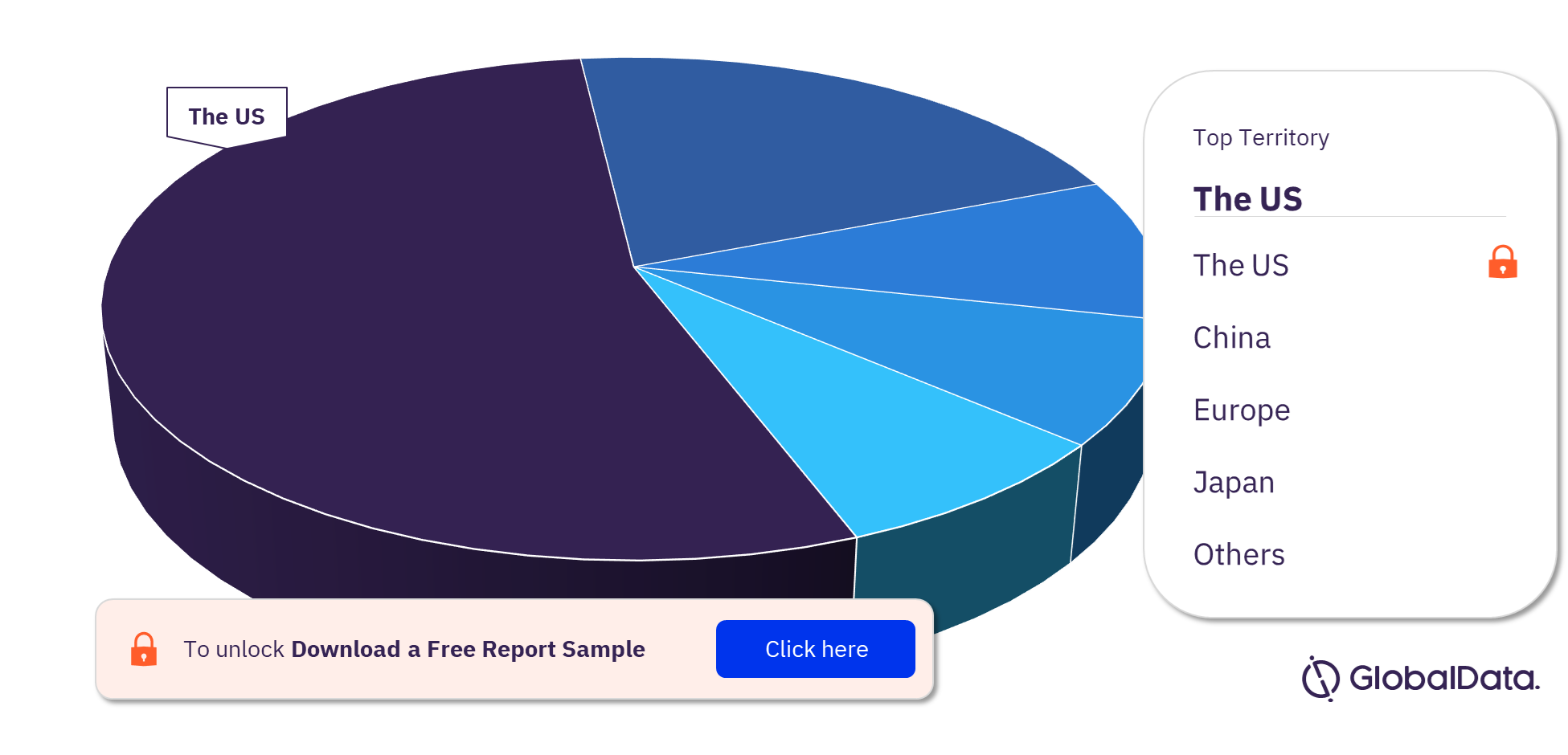
Peripheral Vascular Stents Pipeline Products Market Segmentation by Territories
The key territories with products in the pipeline are the US, China, Europe, Japan, India, New Zealand, the UK, Mauritius, and Canada. As of February 2023, the US has the highest number of products in the pipeline out of them all.
Peripheral Vascular Stents Pipeline Products Market Analysis, by Territories, 2023 (%)
For more territory insights into the Peripheral Vascular Stents pipeline products market, download a free report sample
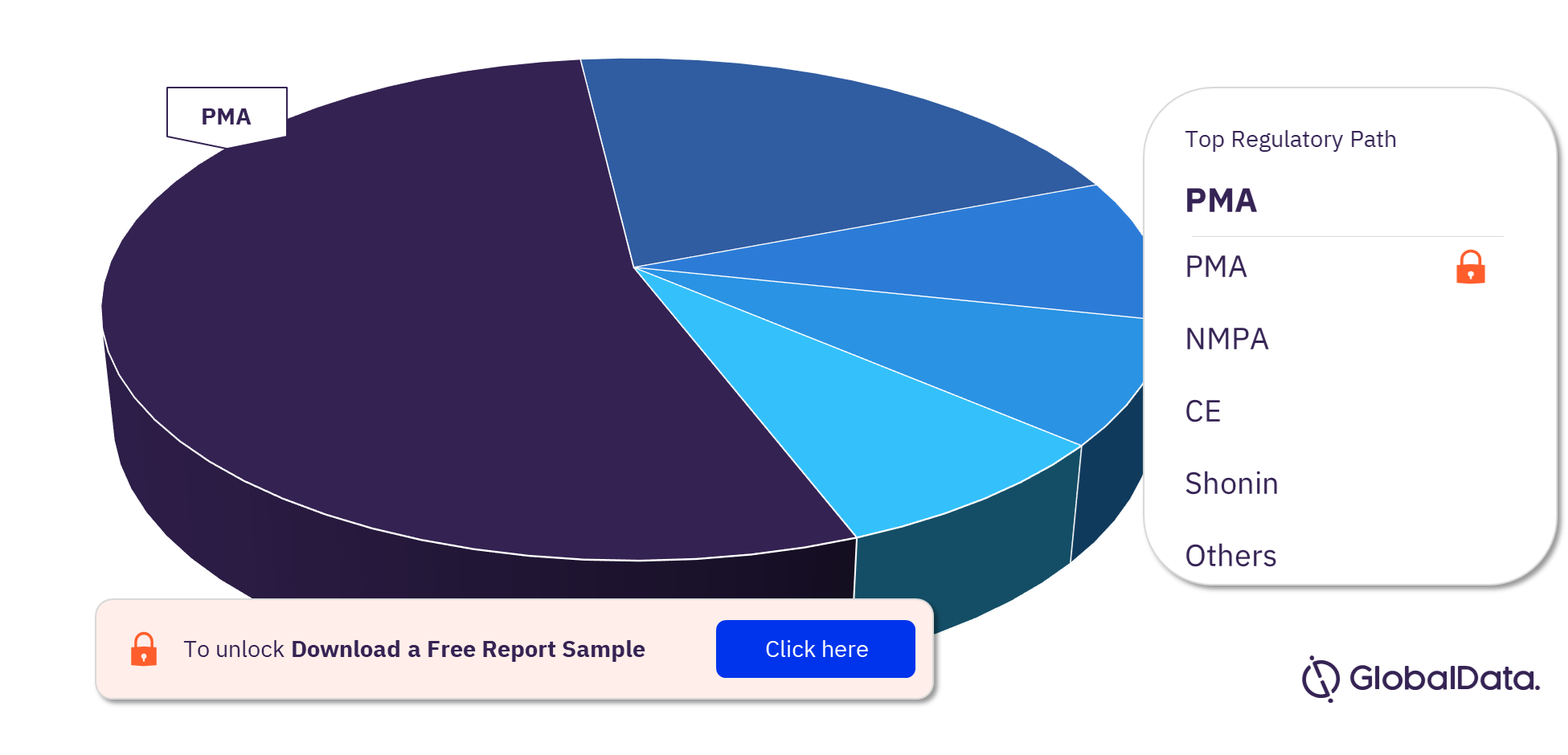
Peripheral Vascular Stents Pipeline Products Market Segmentation by Regulatory Paths
The key regulatory paths followed by the Peripheral Vascular Stents pipeline products market are PMA, NMPA, CE, Shonin, ICAC, 510(k), TGA, UKCA, and MDL. Most of the products follow the PMA pathway to enter the market.
Peripheral Vascular Stents Pipeline Products Market Analysis, by Regulatory Paths, 2023 (%)
For more Peripheral Vascular Stents pipeline products regulatory path insights, download a free report sample
Peripheral Vascular Stents Pipeline Products Market - Competitive Landscape
LLC, Abbott Vascular Inc, Abbott Vascular Inc, Acotec Scientific Holdings Ltd, Acotec Scientific Holdings Ltd, amg International GmbH, AndraTec GmbH, Arterius Ltd, Biotyx Medical (Shenzhen) Co Ltd, and Boston Scientific Corp.
Abbott Vascular Inc: headquartered in California, USA, Abbott Vascular develops, manufactures, and markets cardiovascular devices. The company offers coronary intervention, peripheral intervention, and structural heart products. Abbott Vascular’s peripheral intervention products comprise peripheral guide wires, dilatation catheters, guiding catheters, and stent-grafts. The company provides minimally invasive treatment for mitral regurgitation and other structural defects of the heart. It also offers education and training services for healthcare professionals.
amg International GmbH: Headquartered in Winsen, Germany, amg International is a medical device company that develops, manufactures, and markets stents, stent implantation systems and balloon catheters, and other auxiliary products. It provides coronary vascular intervention products and peripheral vascular intervention products.
Boston Scientific Corp: Headquartered in Massachusetts, USA, Boston Scientific is involved in the development, manufacturing, and commercialization of devices for a range of interventional medical specialties. Boston Scientific serves hospitals, clinics, outpatient facilities, and medical offices across the world. The company has manufacturing facilities in the US, Ireland, Costa Rica, Brazil, Malaysia, Puerto Rico, and Switzerland. It sells products directly and through a network of distributors and dealers in Europe, the Middle East, Africa, Asia Pacific, and the Americas.
For more information on leading players in the Peripheral Vascular Stents pipeline products market, download a free report sample
Peripheral Vascular Stents Pipeline Products Market Report Overview
| Key Segments | Bioabsorbable Stents (BAS), Drug Eluting Stents (DES), Iliac Artery Stents, Tracheobronchial Stent, and Bare Metal Stents (BMS) |
| Key Territories | The US, China, Europe, Japan, India, New Zealand, the UK, Mauritius, and Canada |
| Key Regulatory Paths | PMA, NMPA, CE, Shonin, ICAC, 510(k), TGA, UKCA, and MDL |
| Leading Companies | 3D Biotek LLC, Abbott Vascular Inc, Abbott Vascular Inc, Acotec Scientific Holdings Ltd, Acotec Scientific Holdings Ltd, amg International GmbH, AndraTec GmbH, Arterius Ltd, Biotyx Medical (Shenzhen) Co Ltd, and Boston Scientific Corp |
Scope
This report provides:
- Extensive coverage of the peripheral vascular stents under development.
- Details of major pipeline products which include product description, licensing, collaboration details, and other developmental activities.
- Reviews of the major players involved in the development of peripheral vascular stents and lists all their pipeline projects.
- The coverage of pipeline products based on various stages of development ranging from early development to the approved/issued stage.
- Key clinical trial data of ongoing trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies
- Identify emerging players with potentially strong product portfolio and create effective counter strategies to gain competitive advantage
- Identify and understand important and diverse types of Peripheral Vascular Stents under development
- Develop market-entry and market-expansion strategies
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date
Abbott Vascular Inc
Acotec Scientific Holdings Ltd
amg International GmbH
AndraTec GmbH
Arterius Ltd
Biotyx Medical (Shenzhen) Co Ltd
Boston Scientific Corp
Cardinal Health Inc
Cook Medical Inc
Cordis Corp
CorInnova, Inc.
Cytograft Tissue Engineering Inc (Inactive)
Efemoral Medical LLC
Efferent Labs Inc
Electroformed Stents Inc
Elixir Medical Corp
Endomimetics LLC
FIT Biotech Oy (Inactive)
HeMo Bioengineering Ltd
ID Nest Medical SAS
InspireMD Inc
Intressa Vascular SA
Kyoto Medical Planning Co Ltd
LeoMed, LLC
Lepu Medical Technology (Beijing) Co Ltd
LimFlow SA
Lyra Therapeutics Inc
Meril Life Sciences Pvt Ltd
Micell Technologies Inc
Micro Medical Solutions Inc
Natec Medical Ltd
Nipro Corp
NSVascular, Inc.
OrbusNeich
PeriTec Biosciences Ltd
QualiMed Innovative Medizinprodukte GmbH
Qvanteq AG
Reflow Medical, Inc.
Shanghai Bio-heart Biological Technology Co Ltd
Shanghai Changde Medical Technology Co Ltd
Shanghai MicroPort Endovascular MedTech Co Ltd
Shenzhen Salubris Pharmaceuticals Co Ltd
Suzhou Yinluo Medical Devices Co Ltd
Synergy Flow Ltd
Tepha Inc
TissueGen Inc
Tractivus SL
University of Michigan
University of Pittsburgh
University of Pittsburgh Medical Center
Vascular Bioresorbable Technologies
Vascular Concepts Ltd
Vesper Medical Inc
Vinnova
VueKlar Cardiovascular Ltd (Inactive)
Wake Forest University Health Sciences
Xenogenics Corporation
Zorion Medical
Zylox-Tonbridge Medical Technology Co Ltd
Table of Contents
Table
Figures
Frequently asked questions
-
What are the key segments in the Peripheral Vascular Stents Pipeline Products market?
The key segments in the Peripheral Vascular Stents Pipeline Products market are Bioabsorbable Stents (BAS), Drug Eluting Stents (DES), Iliac Artery Stents, Tracheobronchial Stent, and Bare Metal Stents (BMS).
-
What are the key territories in the Peripheral Vascular Stents pipeline products market?
The US, China, Europe, Japan, India, New Zealand, the UK, Mauritius, and Canada are the key territories with products in the pipeline.
-
What are the key regulatory paths of the Peripheral Vascular Stents pipeline products market?
The key regulatory paths followed by the Peripheral Vascular Stents pipeline products market are PMA, NMPA, CE, Shonin, ICAC, 510(k), TGA, UKCA, and MDL.
-
What are the leading companies in the Peripheral Vascular Stents pipeline products market?
Some of the leading companies in the Peripheral Vascular Stents pipeline products market are 3D Biotek LLC, Abbott Vascular Inc, Abbott Vascular Inc, Acotec Scientific Holdings Ltd, Acotec Scientific Holdings Ltd, amg International GmbH, AndraTec GmbH, Arterius Ltd, Biotyx Medical (Shenzhen) Co Ltd, and Boston Scientific Corp.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Peripheral Vascular Stents reports