Primary Biliary Cholangitis (Primary Biliary Cirrhosis) Drugs in Development by Stages, Target, MoA, RoA, Molecule Type and Key Players, 2022 Update
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Primary biliary cirrhosis is a chronic disease of the liver that slowly destroys the bile ducts within the liver. Symptoms include fatigue, itchy skin, dry eyes, jaundice, swollen feet and ankles, pain in the upper right portion of the abdomen and diarrhea. Predisposing factors include age and history of infections. Treatment includes bile acid sequestrants and liver transplantation.
The Primary Biliary Cholangitis (Primary Biliary Cirrhosis) Drugs in Development market research report provides an overview of the Primary Biliary Cholangitis (Primary Biliary Cirrhosis) pipeline landscape. The report provides comprehensive information on the therapeutics under development for Primary Biliary Cholangitis (Primary Biliary Cirrhosis), complete with analysis by stage of development, drug target, mechanism of action (MoA), route of administration (RoA), and molecule type. The report also covers the descriptive pharmacological action of the therapeutics, its complete research and development history and latest news and press releases. Additionally, the report provides an overview of key players involved in therapeutic development for Primary Biliary Cholangitis (Primary Biliary Cirrhosis) and features dormant and discontinued projects.
What are the targets of the Primary Biliary Cholangitis pipeline drugs market?
Some of the targets of the Primary Biliary Cholangitis pipeline drugs market are Peroxisome Proliferator Activated Receptor Alpha, Bile Acid Receptor, Peroxisome Proliferator Activated Receptor Gamma, Ileal Sodium/Bile Acid Cotransporter, NADPH Oxidase, NADPH Oxidase 4 (Kidney Oxidase 1 or KOX1 or Kidney Superoxide Producing NADPH Oxidase or Renal NAD, Peroxisome Proliferator Activated Receptor Delta, Adenosine Receptor A3, Catenin Beta, and Fibroblast Growth Factor Receptor 1.
Primary Biliary Cholangitis pipeline drugs market, by targets
For more target insights, download a free report sample
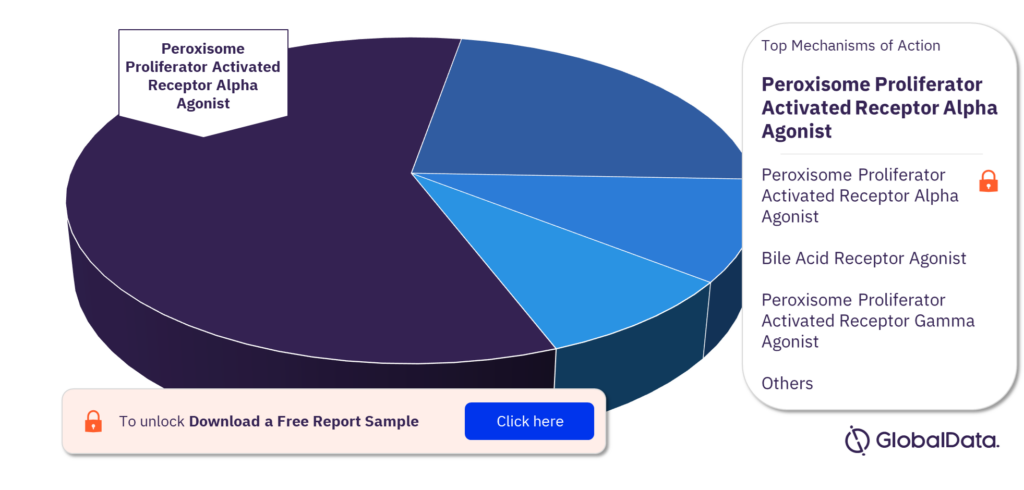
What are the mechanisms of action of the Primary Biliary Cholangitis pipeline drugs market?
Some of the mechanisms of action of the Primary Biliary Cholangitis pipeline drugs market are Peroxisome Proliferator Activated Receptor Alpha Agonist, Bile Acid Receptor Agonist, Peroxisome Proliferator Activated Receptor Gamma Agonist, Ileal Sodium/Bile Acid Cotransporter Inhibitor, NADPH Oxidase 1 Inhibitor, NADPH Oxidase 4 (Kidney Oxidase 1 or KOX1 or Kidney Superoxide Producing NADPH Oxidase or Renal NADInhibitor, Peroxisome Proliferator Activated Receptor Delta Agonist, Adenosine Receptor A3 Antagonist, Catenin Beta 1 Inhibitor, and Fibroblast Growth Factor Receptor 1 Agonist.
Primary Biliary Cholangitis pipeline drugs market, by mechanisms of action
For more mechanisms of action insights, download a free report sample
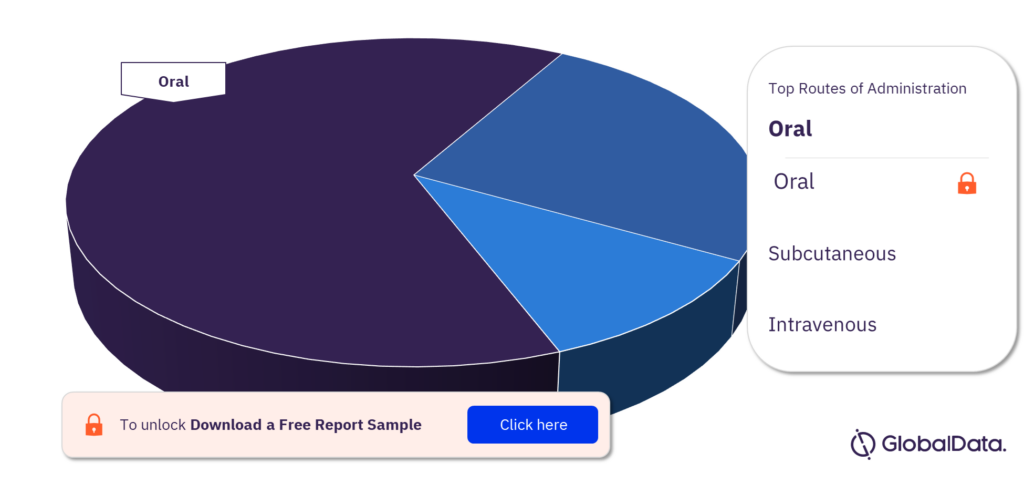
What are the routes of administration in the Primary Biliary Cholangitis pipeline drugs market?
Some of the routes of administration in the Primary Biliary Cholangitis pipeline drugs market are oral, subcutaneous, and intravenous.
Primary Biliary Cholangitis pipeline drugs market, by routes of administrationc
For more routes of administration insights, download a free report sample
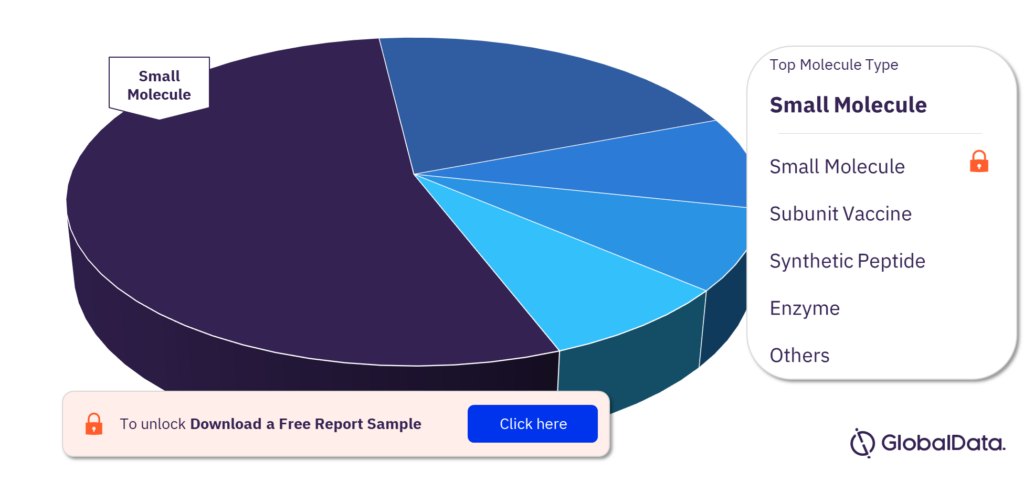
What are the molecule types in the Primary Biliary Cholangitis pipeline drugs market?
The molecule types in the Primary Biliary Cholangitis pipeline drugs market are small molecule, subunit vaccine, synthetic peptide, enzyme, gene-modified cell therapy, monoclonal antibody, oligonucleotide, and recombinant protein.
Primary Biliary Cholangitis pipeline drugs market, by molecule types
For more molecule type insights, download a free report sample
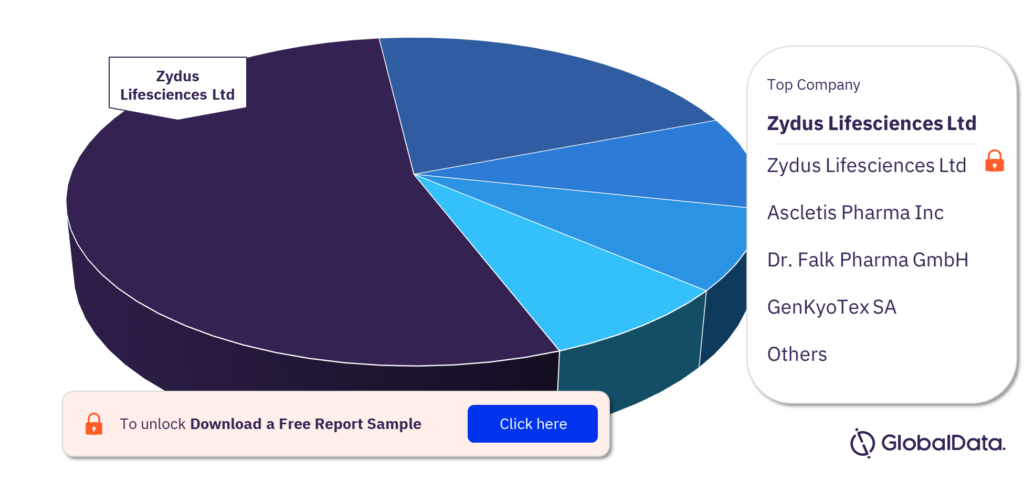
Which are the key companies in the Primary Biliary Cholangitis pipeline drugs market?
Some of the key companies in the Primary Biliary Cholangitis pipeline drugs market are Zydus Lifesciences Ltd, Ascletis Pharma Inc, Dr. Falk Pharma GmbH, GenKyoTex SA, AbbVie Inc, Abcuro Inc, Albireo Pharma Inc, Alexion Pharmaceuticals Inc, Avolynt Inc, and Calliditas Therapeutics AB.
Primary Biliary Cholangitis pipeline drugs market, by key companies
To know more about key companies, download a free report sample
Market report overview
|
Targets |
Peroxisome Proliferator Activated Receptor Alpha, Bile Acid Receptor, Peroxisome Proliferator Activated Receptor Gamma, Ileal Sodium/Bile Acid Cotransporter, NADPH Oxidase, NADPH Oxidase 4 (Kidney Oxidase 1 or KOX1 or Kidney Superoxide Producing NADPH Oxidase or Renal NAD, Peroxisome Proliferator Activated Receptor Delta, Adenosine Receptor A3, Catenin Beta, and Fibroblast Growth Factor Receptor 1 |
| Mechanisms of Action | Peroxisome Proliferator Activated Receptor Alpha Agonist, Bile Acid Receptor Agonist, Peroxisome Proliferator Activated Receptor Gamma Agonist, Ileal Sodium/Bile Acid Cotransporter Inhibitor, NADPH Oxidase 1 Inhibitor, NADPH Oxidase 4 (Kidney Oxidase 1 or KOX1 or Kidney Superoxide Producing NADPH Oxidase or Renal NADInhibitor, Peroxisome Proliferator Activated Receptor Delta Agonist, Adenosine Receptor A3 Antagonist, Catenin Beta 1 Inhibitor, and Fibroblast Growth Factor Receptor 1 Agonist |
| Routes of Administration | Oral, Subcutaneous, and Intravenous |
| Molecule Types | Small Molecule, Subunit Vaccine, Synthetic Peptide, Enzyme, Gene-Modified Cell Therapy, Monoclonal Antibody, Oligonucleotide, and Recombinant Protein |
| Key Companies | Zydus Lifesciences Ltd, Ascletis Pharma Inc, Dr. Falk Pharma GmbH, GenKyoTex SA, AbbVie Inc, Abcuro Inc, Albireo Pharma Inc, Alexion Pharmaceuticals Inc, Avolynt Inc, and Calliditas Therapeutics AB |
Scope
This report provides:
- A snapshot of the global therapeutic landscape of Primary Biliary Cholangitis (Other Diseases).
- Reviews of pipeline therapeutics for Primary Biliary Cholangitis (Other Diseases) by companies and universities/research institutes based on information derived from company and industry-specific sources.
- Pipeline products based on various stages of development ranging from pre-registration to discovery and undisclosed stages.
- Descriptive drug profiles for the pipeline products which include product description, descriptive MoA, R&D brief, licensing and collaboration details & other developmental activities.
- Key players involved in Primary Biliary Cholangitis (Other Diseases) therapeutics and enlists all their major and minor projects.
- Assessment of Primary Biliary Cholangitis (Other Diseases) therapeutics based on drug target, mechanism of action (MoA), route of administration (RoA), and molecule type.
- All the dormant and discontinued pipeline projects.
- Reviews of the latest news related to pipeline therapeutics for Primary Biliary Cholangitis (Other Diseases).
Reasons to Buy
- Procure strategically important competitor information, analysis, and insights to formulate effective R&D strategies.
- Recognize emerging players with potentially strong product portfolio and create effective counter-strategies to gain competitive advantage.
- Find and recognize significant and varied types of therapeutics under development for Primary Biliary Cholangitis (Other Diseases).
- Classify potential new clients or partners in the target demographic.
- Develop tactical initiatives by understanding the focus areas of leading companies.
- Plan mergers and acquisitions meritoriously by identifying key players and its most promising pipeline therapeutics.
- Formulate corrective measures for pipeline projects by understanding Primary Biliary Cholangitis (Other Diseases) pipeline depth and focus of Indication therapeutics.
- Develop and design in-licensing and out-licensing strategies by identifying prospective partners with the most attractive projects to enhance and expand business potential and scope.
- Adjust the therapeutic portfolio by recognizing discontinued projects and understand from the know-how what drove them from pipeline.
Abcuro Inc
Albireo Pharma Inc
Alexion Pharmaceuticals Inc
Ascletis Pharma Inc
Avolynt Inc
Calliditas Therapeutics AB
CAMP4 Therapeutics Corp
Chia Tai Tianqing Pharmaceutical Group Co Ltd
Cour Pharmaceuticals Development Co Inc
CymaBay Therapeutics Inc
Dr. Falk Pharma GmbH
Escient Pharmaceuticals Inc
Future Medicine Co Ltd
Genfit SA
GenKyoTex SA
GentiBio Inc
Gilead Sciences Inc
Hanmi Pharmaceuticals Co Ltd
HighTide Therapeutics Inc
Kissei Pharmaceutical Co Ltd
Kowa Co Ltd
MediGene AG
Mirum Pharmaceuticals Inc
NGM Biopharmaceuticals Inc
Parvus Therapeutics Inc
PRISM Pharma Co Ltd
Selecta Biosciences Inc
Shaanxi Micot Technology Co Ltd
Suzhou Zelgen Biopharmaceutical Co Ltd
Umecrine Cognition AB
Zydus Lifesciences Ltd
Table of Contents
Table
Figures
Frequently asked questions
-
What are the targets of the Primary Biliary Cholangitis pipeline drugs market?
Some of the targets of the Primary Biliary Cholangitis pipeline drugs market are Peroxisome Proliferator Activated Receptor Alpha, Bile Acid Receptor, Peroxisome Proliferator Activated Receptor Gamma, Ileal Sodium/Bile Acid Cotransporter, NADPH Oxidase, NADPH Oxidase 4 (Kidney Oxidase 1 or KOX1 or Kidney Superoxide Producing NADPH Oxidase or Renal NAD, Peroxisome Proliferator Activated Receptor Delta, Adenosine Receptor A3, Catenin Beta, and Fibroblast Growth Factor Receptor 1.
-
What are the mechanisms of action of the Primary Biliary Cholangitis pipeline drugs market?
Some of the mechanisms of action of the Primary Biliary Cholangitis pipeline drugs market are Peroxisome Proliferator Activated Receptor Alpha Agonist, Bile Acid Receptor Agonist, Peroxisome Proliferator Activated Receptor Gamma Agonist, Ileal Sodium/Bile Acid Cotransporter Inhibitor, NADPH Oxidase 1 Inhibitor, NADPH Oxidase 4 (Kidney Oxidase 1 or KOX1 or Kidney Superoxide Producing NADPH Oxidase or Renal NADInhibitor, Peroxisome Proliferator Activated Receptor Delta Agonist, Adenosine Receptor A3 Antagonist, Catenin Beta 1 Inhibitor, and Fibroblast Growth Factor Receptor 1 Agonist.
-
What are the routes of administration in the Primary Biliary Cholangitis pipeline drugs market?
Some of the routes of administration in the Primary Biliary Cholangitis pipeline drugs market are oral, subcutaneous, and intravenous.
-
What are the molecule types in the Primary Biliary Cholangitis pipeline drugs market?
The molecule types in the Primary Biliary Cholangitis pipeline drugs market are small molecule, subunit vaccine, synthetic peptide, enzyme, gene-modified cell therapy, monoclonal antibody, oligonucleotide, and recombinant protein.
-
Which are the key companies in the Primary Biliary Cholangitis pipeline drugs market?
Some of the key companies in the Primary Biliary Cholangitis pipeline drugs market are Zydus Lifesciences Ltd, Ascletis Pharma Inc, Dr. Falk Pharma GmbH, GenKyoTex SA, AbbVie Inc, Abcuro Inc, Albireo Pharma Inc, Alexion Pharmaceuticals Inc, Avolynt Inc, and Calliditas Therapeutics AB.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Other Diseases reports