Sickle Cell Disease – Global Clinical Trials Review, H2, 2021
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
GlobalData’s clinical trial report provides data on the sickle cell disease clinical trial scenario. This report provides top-line data relating to the clinical trials on Sickle Cell Disease. The report includes an overview of trials count and their average enrolment in top countries conducted globally. The report includes coverage of disease clinical trials by region and top countries, trial phase, trial status, end points status, and sponsor type. The report also provides prominent drug names and trial counts for in-progress trials (based on the number of ongoing trials).
The Sickle Cell Disease clinical trial report consists of 696 trials. Trials conducted by companies in Phase IV, Phase III, Phase II, Phase I, and Phase 0 stand at 21, 59, 128, 115, and 1 respectively. Similarly, the trials with different status compile of Completed – 350, Ongoing – 178, Planned – 35, Suspended – 4, Terminated – 75, and Withdrawn – 54. Out of 350 completed trials, 182 trials have achieved end points.
What are the market dynamics of the global sickle cell disease therapeutics clinical trials sector?
The number of sickle cell disease clinical trials conducted globally, has increased by 2% for the period 2016-2020. The average number of patients enrolled was highest in the year 2017. As of November 2021, out of 350 completed trials, 243 trials have results and over 75% of trials reached end points. There were 178 clinical trials in progress and 350 trials were completed. The trials that were terminated/suspended or withdrawn accounted for 133. This was due to the lack of efficacy, safety, and lack of accrual of subjects. As of November 2021, the majority of the trials were successfully completed. There were 178 clinical trials in progress of which 39% (69 trials) were in Phase II study. There were 280 clinical trials in Phase II, of which 69 clinical trials were in progress for the time period 1995-2021. Institutions were the leading sponsor for sickle cell disease clinical trials followed by companies and governments.
What are the top regions and countries in the global sickle cell disease therapeutics clinical trials sector?
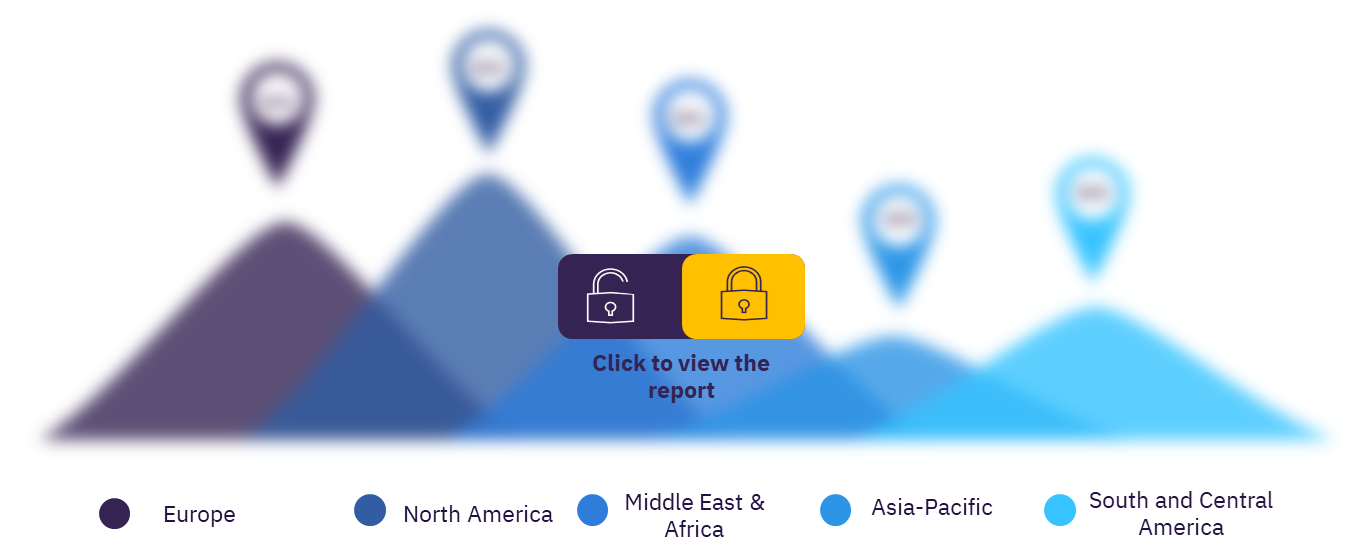
In total there were 696 clinical trials conducted on sickle cell disease, as of November 2021, of these 431 clinical trials were in North America. More than 55% of the clinical trials were conducted in North America.
Asia-Pacific: As of November 2021, in the Asia-Pacific region, India has the highest number of sickle cell disease clinical trials followed by Australia, Thailand, China, and Malaysia.
Europe: Among the European countries, the UK has the highest number of sickle cell disease clinical trials, as of November 2021, followed by France, Italy, the Netherlands, and Germany.
North America: In the country-wise analysis, the US has the highest number of sickle cell disease clinical trials, as of November 2021 followed by Canada.
Middle East and Africa: Lebanon has the highest number of sickle cell disease clinical trials, as of November 2021 followed by Egypt, Nigeria, Kenya, and Ghana.
Central and South America: Brazil has the highest number of sickle cell disease clinical trials, in the region followed by Jamaica, Colombia, Panama, and the Dominican Republic
G7 countries: Among the G7 (The US, the UK, Germany, France, Italy, Canada, and Japan) countries, The US has the highest proportion of sickle cell disease to hematological disorders clinical trials as of November 2021. In total there were 648 clinical trials conducted on Sickle Cell Disease, as of November 2021 in G7 Countries, of these 423 clinical trials were in the US. There were 141 clinical trials in progress and 241 trials are completed. The trials that are terminated/suspended or withdrawn accounted for 92. This was due to the lack of efficacy, safety, and lack of accrual of subjects.
E7 countries: Among the E7 (Brazil, Russia, India, China, Mexico, Turkey, and Indonesia) countries, Brazil has the highest proportion of sickle cell disease to hematological disorders clinical trials as of November 2021. In total there were 76 clinical trials conducted on Sickle Cell Disease, as of November 2021 in E7 Countries, of these 29 clinical trials were in Brazil. There were 15 clinical trials in progress and 27 trials were completed. The trials that were terminated/suspended or withdrawn accounted for 12.
Global sickle cell disease therapeutics clinical trials sector, by regions
For more regional insights, download a free report sample
Which are the key companies in the global sickle cell disease therapeutics clinical trials sector?
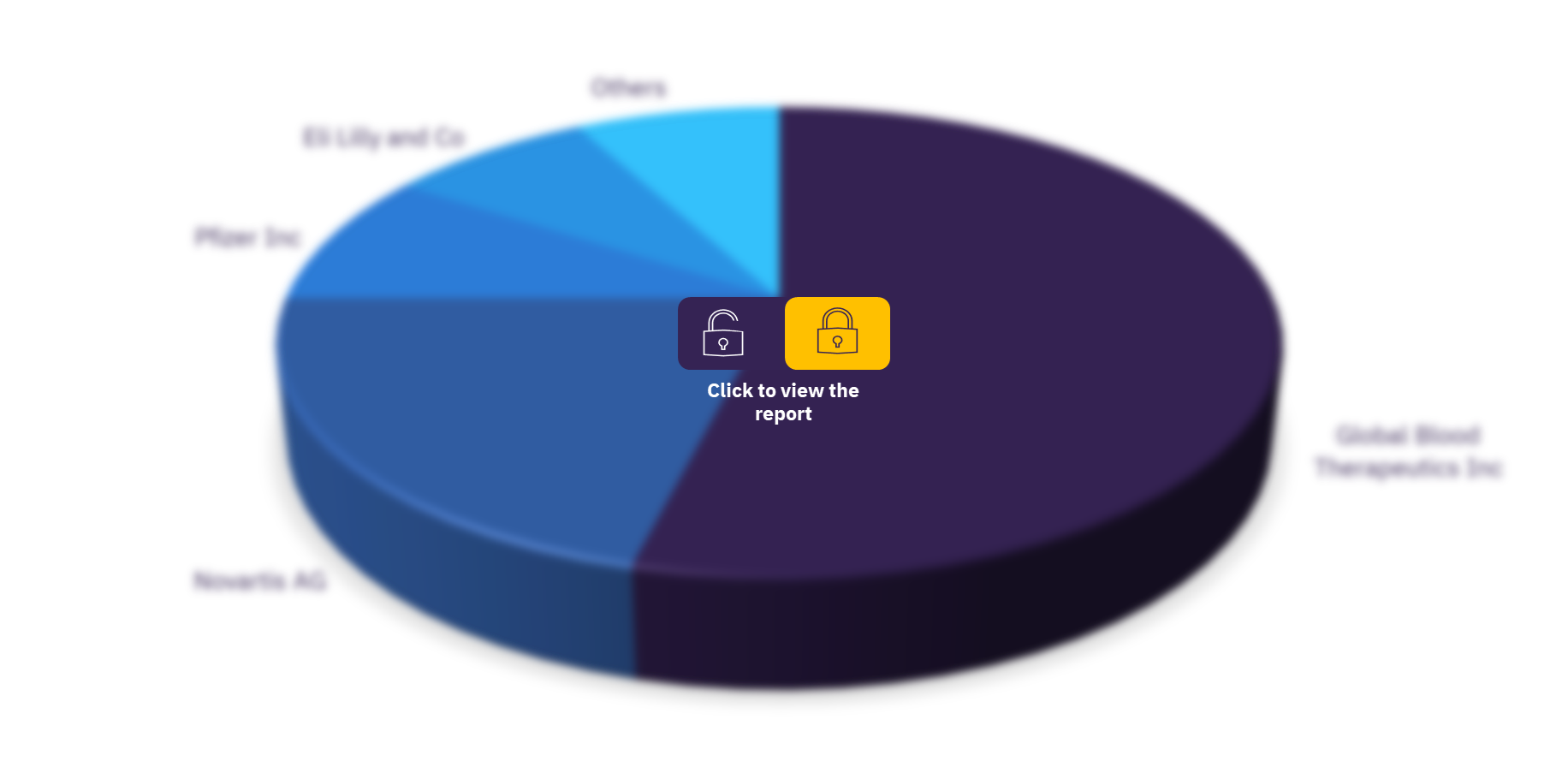
As of November 2021, Global Blood Therapeutics Inc has conducted the highest number of sickle cell disease clinical trials followed by Novartis AG. Other major companies in the sector are Pfizer Inc, Eli Lilly and Co, GlycoMimetics Inc, IQVIA Holdings Inc, bluebird bio Inc., Mast Therapeutics Inc (Inactive), Prolong Pharmaceuticals LLC, and Agios Pharmaceuticals Inc.
Global sickle cell disease therapeutics clinical trials sector, by key companies
To know more about key companies, download a free report sample
Market report scope
| Key countries | The US, the UK, France, Canada, Italy, the Netherlands, Brazil, Lebanon, Egypt, and Nigeria |
| Key companies | Global Blood Therapeutics Inc, Novartis AG, Pfizer Inc, Eli Lilly and Co, GlycoMimetics Inc, IQVIA Holdings Inc, bluebird bio Inc., Mast Therapeutics Inc (Inactive), Prolong Pharmaceuticals LLC, and Agios Pharmaceuticals Inc. |
Scope
- The report provides a snapshot of the global clinical trials landscape.
- Report provides top-level data related to the clinical trials by Region, Country (G7 & E7), Trial Status, Trial Phase, Sponsor Type, and End point status.
- The report reviews the top companies involved and enlists all trials (Trial title, Phase, and Status) pertaining to the company.
- The report provides all the unaccomplished trials (Terminated, Suspended, and Withdrawn) with the reason for un-accomplishment.
- The Report provides enrollment trends for the past five years.
- Report provides the latest news for the past three months.
Reasons to Buy
- Assists in formulating key business strategies with regard to investment.
- Helps in identifying prominent locations for conducting clinical trials which saves time and cost.
- Provides top-level analysis of the Global Clinical Trials Market which helps in identifying key business opportunities.
- Supports understanding of trials count and enrolment trends by country in the global therapeutics market.
- Aids in interpreting the success rates of clinical trials by providing a comparative scenario of completed and uncompleted (terminated, suspended, or withdrawn) trials.
- Facilitates clinical trial assessment of the indication on a global, regional, and country-level.
Novartis AG
Pfizer Inc
Eli Lilly and Co
GlycoMimetics Inc
IQVIA Holdings Inc
bluebird bio Inc
Mast Therapeutics Inc (Inactive)
Prolong Pharmaceuticals LLC
Agios Pharmaceuticals Inc
Table of Contents
Table
Figures
Frequently asked questions
-
What are the key countries in the global sickle cell disease therapeutics clinical trials sector?
The key countries in the global sickle cell disease therapeutics clinical trials sector are the US, the UK, France, Canada, Italy, the Netherlands, Brazil, Lebanon, Egypt, and Nigeria.
-
Which are the key companies in the global sickle cell disease therapeutics clinical trials sector?
The key companies in the global sickle cell disease therapeutics clinical trials sector are Global Blood Therapeutics Inc, Novartis AG, Pfizer Inc, Eli Lilly and Co, GlycoMimetics Inc, IQVIA Holdings Inc, bluebird bio-Inc., Mast Therapeutics Inc (Inactive), Prolong Pharmaceuticals LLC, and Agios Pharmaceuticals Inc.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Hematological Disorders reports