Spinal Cord Stimulators (SCS) – Pipeline Products by Stage of Development 13
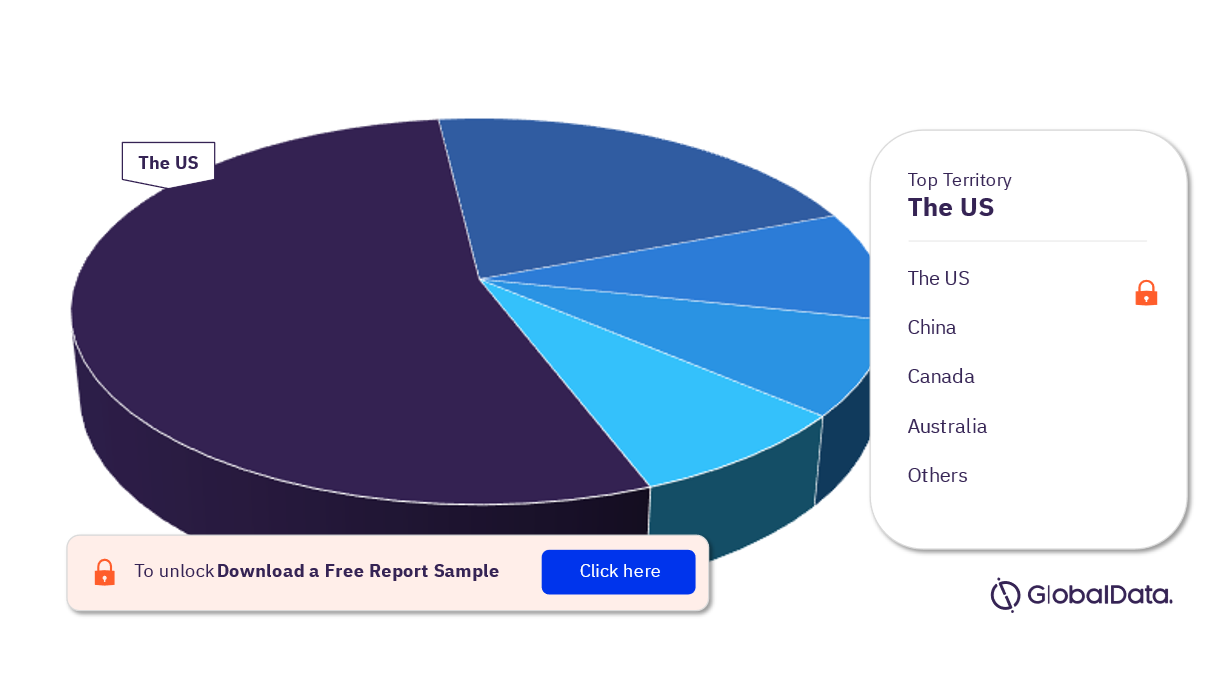
Spinal Cord Stimulators (SCS) – Pipeline Products by Territory 14
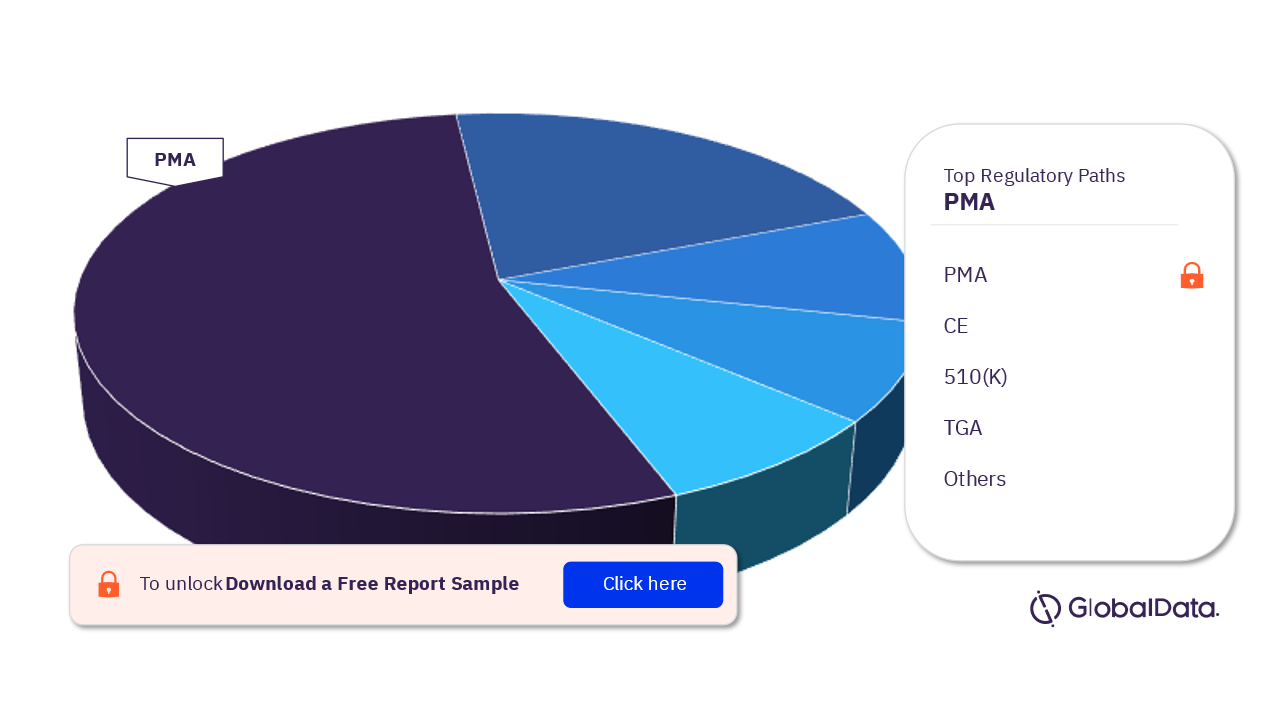
Spinal Cord Stimulators (SCS) – Pipeline Products by Regulatory Path 15
Spinal Cord Stimulators (SCS) – Pipeline Products by Estimated Approval Date 16
Spinal Cord Stimulators (SCS) – Ongoing Clinical Trials 17
Spinal Cord Stimulators (SCS) Companies – Pipeline Products by Stage of Development 18
Spinal Cord Stimulators (SCS) – Pipeline Products by Stage of Development 20
Beijing Leading Medical Valley Technology Development Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 22
Spinal Cord Nerve Stimulation Device – Product Status 22
Spinal Cord Nerve Stimulation Device – Product Description 22
Beijing Lingchuang Yigu Technology Development Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 23
Neural Electrical Stimulation Device – Pain – Product Status 23
Neural Electrical Stimulation Device – Pain – Product Description 23
Boston Scientific Corp Pipeline Products & Ongoing Clinical Trials Overview 24
Next Generation SCS System – Product Status 24
Next Generation SCS System – Product Description 24
WaveWriter Spinal Cord Stimulator (SCS) System – Non-Surgical Back Pain – Product Status 25
WaveWriter Spinal Cord Stimulator (SCS) System – Non-Surgical Back Pain – Product Description 25
Boston Scientific Corp – Ongoing Clinical Trials Overview 26
WaveWriter Spinal Cord Stimulator (SCS) System – Non-Surgical Back Pain – A Randomized Controlled Study to Evaluate the Safety and Effectiveness of Boston Scientific Spinal Cord Stimulation (SCS) Systems in the Treatment of Chronic Low Back and/or Leg Pain with No Prior Surgeries 27
Cerevast Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 28
Transdermal Electrical Neurostimulation Device – Product Status 28
Transdermal Electrical Neurostimulation Device – Product Description 28
Direct Spinal Therapeutics, Inc. Pipeline Products & Ongoing Clinical Trials Overview 29
Iowa-Patch System – Product Status 29
Iowa-Patch System – Product Description 29
Duke University Pipeline Products & Ongoing Clinical Trials Overview 30
Spinal Cord Stimulator – Product Status 30
Spinal Cord Stimulator – Product Description 30
Hangzhou Shenluo Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 31
Neuromodulation Device – Epilepsy – Product Status 31
Neuromodulation Device – Epilepsy – Product Description 31
Imperial College London Pipeline Products & Ongoing Clinical Trials Overview 32
Wireless Intraspinal Microstimulation System – Product Status 32
Wireless Intraspinal Microstimulation System – Product Description 32
Lawson Health Research Institute Pipeline Products & Ongoing Clinical Trials Overview 33
Spinal Implant – Parkinson’s Disease – Product Status 33
Spinal Implant – Parkinson’s Disease – Product Description 33
Meagan Medical Inc. Pipeline Products & Ongoing Clinical Trials Overview 34
DISCSS IFC System – Product Status 34
DISCSS IFC System – Product Description 34
Medtronic Inc Pipeline Products & Ongoing Clinical Trials Overview 35
Inceptiv SCS – Product Status 35
Inceptiv SCS – Product Description 35
Medtronic Inc – Ongoing Clinical Trials Overview 36
Inceptiv SCS – Evaluation of Long-term Patient Experience with a Medtronic Closed-Loop Spinal Cord Stimulation System 37
Medtronic Plc Pipeline Products & Ongoing Clinical Trials Overview 38
Intellis – Painful Diabetic Neuropathy – Product Status 38
Intellis – Painful Diabetic Neuropathy – Product Description 38
Intellis – Upper Limb And Neck – Product Status 39
Intellis – Upper Limb And Neck – Product Description 39
Micro-Leads Inc Pipeline Products & Ongoing Clinical Trials Overview 40
HD64 Implantable Therapy System – Product Status 40
HD64 Implantable Therapy System – Product Description 40
Intelligent Spinal Interface – Product Status 41
Intelligent Spinal Interface – Product Description 41
MicroTransponder Inc Pipeline Products & Ongoing Clinical Trials Overview 42
SAINT System – Chronic Pain – Product Status 42
SAINT System – Chronic Pain – Product Description 42
Neuronoff Inc Pipeline Products & Ongoing Clinical Trials Overview 43
Injectrode – Product Status 43
Injectrode – Product Description 43
Neuronoff Inc – Ongoing Clinical Trials Overview 44
Injectrode – Basic Assessment of Safety and Minimally Invasive Stimulation via Injectrode 45
NeuroOne Medical Technologies Corp Pipeline Products & Ongoing Clinical Trials Overview 46
Spinal Cord Stimulation System – Chronic Back Pain – Product Status 46
Spinal Cord Stimulation System – Chronic Back Pain – Product Description 46
Nevro Corp Pipeline Products & Ongoing Clinical Trials Overview 47
Omnia Upgrade – Product Status 48
Omnia Upgrade – Product Description 48
Senza HF10 Therapy System – Back Pain – Product Status 48
Senza HF10 Therapy System – Back Pain – Product Description 49
Senza HF10 Therapy System – Chronic Abdominal Pain – Product Status 49
Senza HF10 Therapy System – Chronic Abdominal Pain – Product Description 49
Senza HF10 Therapy System – Chronic Migraine – Product Status 50
Senza HF10 Therapy System – Chronic Migraine – Product Description 50
Senza HF10 Therapy System – Chronic Neck Pain – Product Status 50
Senza HF10 Therapy System – Chronic Neck Pain – Product Description 51
Senza HF10 Therapy System – CPSP – Product Status 51
Senza HF10 Therapy System – CPSP – Product Description 51
Senza HF10 Therapy System – Leg Pain – Product Status 52
Senza HF10 Therapy System – Leg Pain – Product Description 52
Senza HF10 Therapy System – Opioid Reduction – Product Status 52
Senza HF10 Therapy System – Opioid Reduction – Product Description 53
Senza HF10 Therapy System – Painful Diabetic Neuropathy – Product Status 53
Senza HF10 Therapy System – Painful Diabetic Neuropathy – Product Description 53
Senza HF10 Therapy System – Pelvic Pain – Product Status 54
Senza HF10 Therapy System – Pelvic Pain – Product Description 54
Senza HF10 Therapy System – Peripheral Polyneuropathy – Product Status 54
Senza HF10 Therapy System – Peripheral Polyneuropathy – Product Description 55
Senza HF10 Therapy System – Post-Surgical Pain – Product Status 55
Senza HF10 Therapy System – Post-Surgical Pain – Product Description 55
Senza HF10 Therapy System – Rescue Therapy – Product Status 56
Senza HF10 Therapy System – Rescue Therapy – Product Description 56
Senza HF10 Therapy System – Thoracic Pain – Product Status 56
Senza HF10 Therapy System – Thoracic Pain – Product Description 57
Senza HF10 Therapy System – Upper Extremity Pain – Product Status 57
Senza HF10 Therapy System – Upper Extremity Pain – Product Description 57
Senza HF10 Therapy System – Upper Limb Pain – Product Status 58
Senza HF10 Therapy System – Upper Limb Pain – Product Description 58
Senza HFX iQ Spinal Cord Stimulation System – Product Status 58
Senza HFX iQ Spinal Cord Stimulation System – Product Description 59
Nevro Corp – Ongoing Clinical Trials Overview 60
Senza HF10 Therapy System – Painful Diabetic Neuropathy – A Post-market, Multicenter, Prospective, Global Clinical Study to Evaluate the Real-world Experience of Spinal Cord Stimulation That Includes 10 kHz in the Management of Chronic Intractable Pain Associated with Diabetic Neuropathy 61
Senza HF10 Therapy System – Painful Diabetic Neuropathy – A Post-market, Multicenter, Prospective, Randomized Clinical Trial Comparing 10 kHz Spinal Cord Stimulation (HF10 Therapy) Combined with Conventional Medical Management to Conventional Medical Management Alone in the Treatment of Chronic, Intractable, Neuropathic Limb Pain 61
Senza HF10 Therapy System – Painful Diabetic Neuropathy – PDN-SENSORY: A Multi-Center Randomized Controlled Trial to Evaluate Pain and Neurological Function with 10 kHz SCS in Treatment of Painful Diabetic Neuropathy 61
Senza HF10 Therapy System – Back Pain – A Multi-center, Prospective, Pragmatic, Randomized, Controlled Clinical Trial to Compare HF10 Therapy to Conventional Medical Management in the Treatment of Non-surgical Refractory Back Pain 62
Senza HF10 Therapy System – Pelvic Pain – A Prospective Clinical Study to Assess the Spinal Cord Stimulation System in the Treatment of Chronic Pelvic Pain 63
Nexstim Plc Pipeline Products & Ongoing Clinical Trials Overview 64
Navigated Brain Therapy System – Chronic Spinal Cord Injury – Product Status 64
Navigated Brain Therapy System – Chronic Spinal Cord Injury – Product Description 64
Nuvectra Corp (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 65
Algovita SCS System – Full Body MRI – Product Status 65
Algovita SCS System – Full Body MRI – Product Description 65
Onward Medical NV Pipeline Products & Ongoing Clinical Trials Overview 66
Go-2 Targeted Epidural Spinal Stimulation Device – Product Status 66
Go-2 Targeted Epidural Spinal Stimulation Device – Product Description 66
Onward Medical NV – Ongoing Clinical Trials Overview 67
Go-2 Targeted Epidural Spinal Stimulation Device – Efficacy of Spinal Epidural Electrical Stimulation (EES) in Combination with Robot-assisted Neurorehabilitation in Patients with Spinal Cord Injury (STIMO) 68
Go-2 Targeted Epidural Spinal Stimulation Device – Targeting Improvements in Bowel Function and Quality of Life Using Epidural Stimulation and Training After Severe Spinal Cord Injury 68
Onward Technologies Ltd Pipeline Products & Ongoing Clinical Trials Overview 69
ARC-BCI – Mobility – Product Status 69
ARC-BCI – Mobility – Product Description 70
ARC-BCI – Upper Limb – Product Status 70
ARC-BCI – Upper Limb – Product Description 70
ARC-BSI – Product Status 71
ARC-BSI – Product Description 71
ARC-EX – Product Status 71
ARC-EX – Product Description 72
ARC-EX – Bladder Control – Product Status 72
ARC-EX – Bladder Control – Product Description 72
ARC-EX – Mobility – Product Status 73
ARC-EX – Mobility – Product Description 73
ARC-EX – Sexual Function – Product Status 73
ARC-EX – Sexual Function – Product Description 74
ARC-IM – Blood Pressure And Trunk Control – Product Status 74
ARC-IM – Blood Pressure And Trunk Control – Product Description 74
ARC-IM – Parkinson’s Disease – Product Status 75
ARC-IM – Parkinson’s Disease – Product Description 75
ARCEX Neurostimulator – Product Status 75
ARCEX Neurostimulator – Product Description 76
Onward Technologies Ltd – Ongoing Clinical Trials Overview 77
ARC-EX – Activity Dependent Rehabilitation Via Transcutaneous Electrical Spinal Stimulation to Restore Upper Extremity Functions in Spinal Cord Injury 78
ARC-IM – Blood Pressure And Trunk Control – Epidural Electrical Stimulation to Restore Hemodynamic Stability and Trunk Control in People with Spinal Cord Injury 79
Quantum Nanostim LLC Pipeline Products & Ongoing Clinical Trials Overview 80
Clone System – Product Status 80
Clone System – Product Description 80
Reach Neuro Inc Pipeline Products & Ongoing Clinical Trials Overview 81
Avantis Device – Product Status 81
Avantis Device – Product Description 81
Rowan University Pipeline Products & Ongoing Clinical Trials Overview 82
Spinal Cord Stimulation Device – Chronic Pain – Product Status 82
Spinal Cord Stimulation Device – Chronic Pain – Product Description 82
Saluda Medical Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 83
Evoke Spinal Cord Stimulator System – Product Status 83
Evoke Spinal Cord Stimulator System – Product Description 83
Saluda Medical Pty Ltd – Ongoing Clinical Trials Overview 84
Evoke Spinal Cord Stimulator System – A Feasibility Study Evaluating Saluda Medical’s Spinal Cord Stimulation System Incorporating Feedback Control to Treat Patients with Chronic Pain of Upper Limb and/or Neck 85
Evoke Spinal Cord Stimulator System – A prospective Feasibility Study Examining the Ability of Closed-loop Spinal Cord Stimulation to Reduce Lower Limb Spasticity in Children Living with Cerebral Palsy: “The Liberty Trial” 85
Evoke Spinal Cord Stimulator System – A Prospective Study to Evaluate the Long-term Effectiveness, Safety, and Performance of the Saluda Medical’s Evoke Closed-Loop Spinal Cord Stimulation System to Treat Patients with Chronic Pain of the Trunk and/or Limbs 85
Evoke Spinal Cord Stimulator System – A Prospective, Multicentre, Single-arm Feasibility Study Examining Novel Treatment Delivery of the Evoke Spinal Cord Stimulator (SCS) System to Treat Patients with Chronic Pain of the Trunk And/or Limbs 86
Evoke Spinal Cord Stimulator System – EVOKE ECAP-Controlled Lead Placement and Programming in Chronic Pain Patients 86
Soin Neuroscience Inc Pipeline Products & Ongoing Clinical Trials Overview 87
Spinal Cord Stimulation System – Product Status 87
Spinal Cord Stimulation System – Product Description 87
Soterix Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 88
1×1 tDCS Device – Product Status 88
1×1 tDCS Device – Product Description 89
tSDCS Cervical Strap – Product Status 89
tSDCS Cervical Strap – Product Description 89
tSDCS Lumbar BODYstrap – Product Status 90
tSDCS Lumbar BODYstrap – Product Description 90
tSDCS Shoulder Strap – Product Status 90
tSDCS Shoulder Strap – Product Description 91
Soterix Medical Inc – Ongoing Clinical Trials Overview 92
1×1 tDCS Device – Altering Multitasking Behavior Using Low Current Brain Stimulation 93
1×1 tDCS Device – Effects of tDCS on Post-stroke Fatigue and Inflammation 93
1×1 tDCS Device – Effects of Transcranial Direct Current Stimulation (tDCS) in Spoken and Written Production in Primary Progressive Aphasia (PPA) 93
1×1 tDCS Device – Effects of Transcranial Direct Current Stimulation (tDCS) on Motor Function in Schizophrenia Patients and Individuals at Risk for Psychotic Onset 94
1×1 tDCS Device – Impact of Neuromodulation on Language Impairments in Stroke Patients: A Multimodal Double-blind Randomized Controlled Study 94
1×1 tDCS Device – Non-invasive Neuromodulation to Enhance Targeted Cognitive Remediation in Older Adults With Depression 94
1×1 tDCS Device – Phase II Clinical Trial of Transcranial Direct Current Stimulation in the Treatment of Primary Progressive Aphasia 95
1×1 tDCS Device – Pragmatic Trial of Remote tDCS and Somatosensory Training for Phantom Limb Pain with Machine Learning to Predict Treatment Response 95
1×1 tDCS Device – SMART Stimulation: Precision Neuromodulation of Cognition in Older Adults 95
1×1 tDCS Device – The Combined Effect of Robotic Rehabilitation and Transcranial Direct Current Stimulation on Proprioception in Chronic Stroke: a Pilot Study 96
1×1 tDCS Device – Tolerability of Transcranial Direct Current Stimulation in Pediatric Stroke Survivors 96
1×1 tDCS Device – Transcranial Direct Current Stimulation for Gait Recovery Following Stroke 96
1×1 tDCS Device – Using tDCS in Speech-based Stroke Rehabilitation 97
SpineX Pipeline Products & Ongoing Clinical Trials Overview 98
SCiP – Product Status 98
SCiP – Product Description 98
Scone Device – Product Status 99
Scone Device – Product Description 99
SpineX – Ongoing Clinical Trials Overview 100
Scone Device – Noninvasive Spinal Neuromodulation Enables Locomotor Recovery in Individuals with Cerebral Palsy 101
Scone Device – The Effects of Transcutaneous Electrical Stimulation on Arm Functions in Individuals with Cervical Spinal Cord Injury 101
SCiP – Spinal Cord Innovation in Pediatrics (SCiP) to Treat SensoriMotor Function in Children Cerebral Palsy: Protocol for a Randomized Controlled Trial 102
SCiP – Transcutaneous Stimulation and Mobility Device Use for Individuals with Neurologic Conditions 102
Sree Chitra Tirunal Institute for Medical Sciences & Technology Pipeline Products & Ongoing Clinical Trials Overview 103
Spinal Cord Stimulator – Chronic Pain – Product Status 103
Spinal Cord Stimulator – Chronic Pain – Product Description 103
St. Jude Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 104
Next Generation Implantable SCS System – Primary Cell – Product Status 104
Next Generation Implantable SCS System – Primary Cell – Product Description 104
Next Generation Implantable SCS System – Rechargeable – Product Status 105
Next Generation Implantable SCS System – Rechargeable – Product Description 105
Proclaim Rechargeable Model – Product Status 105
Proclaim Rechargeable Model – Product Description 105
Proclaim Upgrade HC – Product Status 106
Proclaim Upgrade HC – Product Description 106
Proclaim Upgrade LC – Product Status 106
Proclaim Upgrade LC – Product Description 107
Stimdia Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 108
pdSTIM System – Product Status 108
pdSTIM System – Product Description 108
SynerFuse Inc Pipeline Products & Ongoing Clinical Trials Overview 109
NeuroFuse DRG System – Product Status 109
NeuroFuse DRG System – Product Description 109
SynerFuse Inc – Ongoing Clinical Trials Overview 110
NeuroFuse DRG System – Pilot Trial to Investigate NeuroFuse DRG Therapy in Patients with Chronic Lower Back Pain 111
University of California Los Angeles Pipeline Products & Ongoing Clinical Trials Overview 112
32-Electrode Neurostimulator – Product Status 112
32-Electrode Neurostimulator – Product Description 112
Spinal Cord Electrical Stimulator – Product Status 113
Spinal Cord Electrical Stimulator – Product Description 113
University of Cambridge Pipeline Products & Ongoing Clinical Trials Overview 114
Inflatable Spinal Implant – Product Status 114
Inflatable Spinal Implant – Product Description 114
University of Minnesota Pipeline Products & Ongoing Clinical Trials Overview 115
Spinal Cord Stimulation Device – Product Status 115
Spinal Cord Stimulation Device – Product Description 115
University of Minnesota – Ongoing Clinical Trials Overview 116
Spinal Cord Stimulation Device – Epidural Stimulation for Spinal Cord Injury 117
Wavegate Corp Pipeline Products & Ongoing Clinical Trials Overview 118
StimuLux – Product Status 118
StimuLux – Product Description 118
WISE s.r.l. Pipeline Products & Ongoing Clinical Trials Overview 119
SCS EXPERT – Product Status 119
SCS EXPERT – Product Description 119
Glossary 146
![]()