Spinal Fusion – Pipeline Products by Stage of Development 28
Spinal Fusion – Pipeline Products by Segment 29
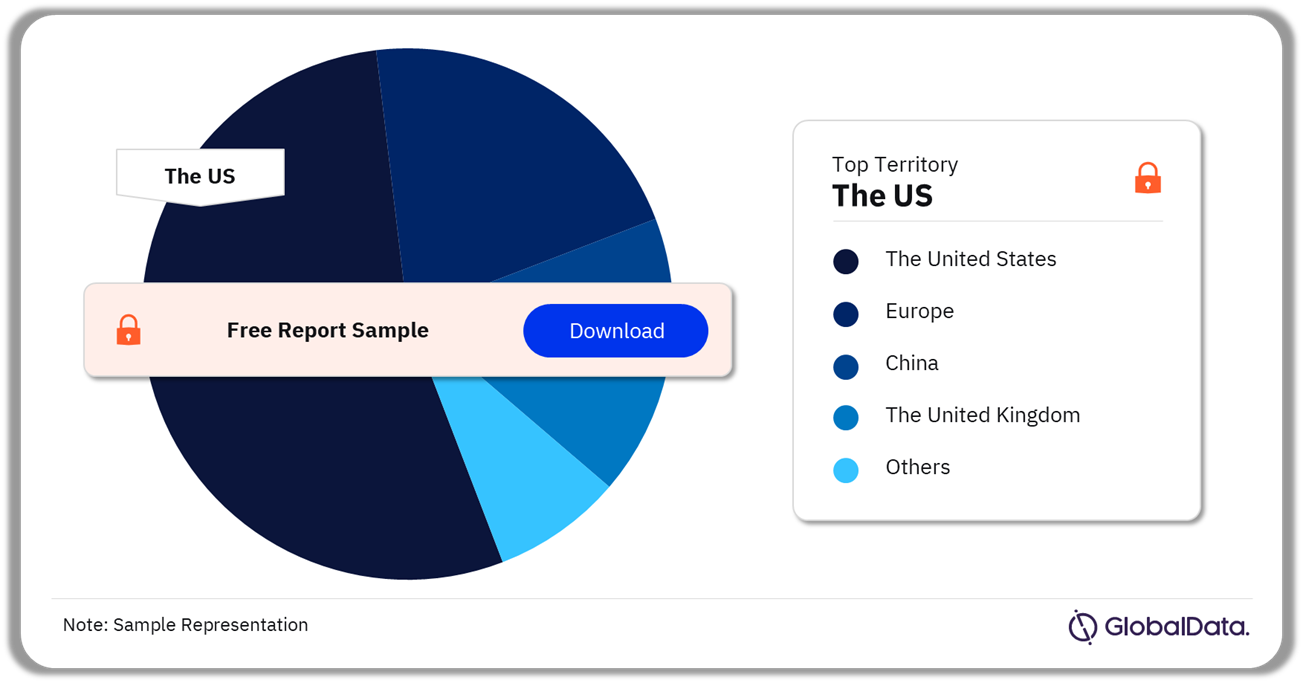
Spinal Fusion – Pipeline Products by Territory 30
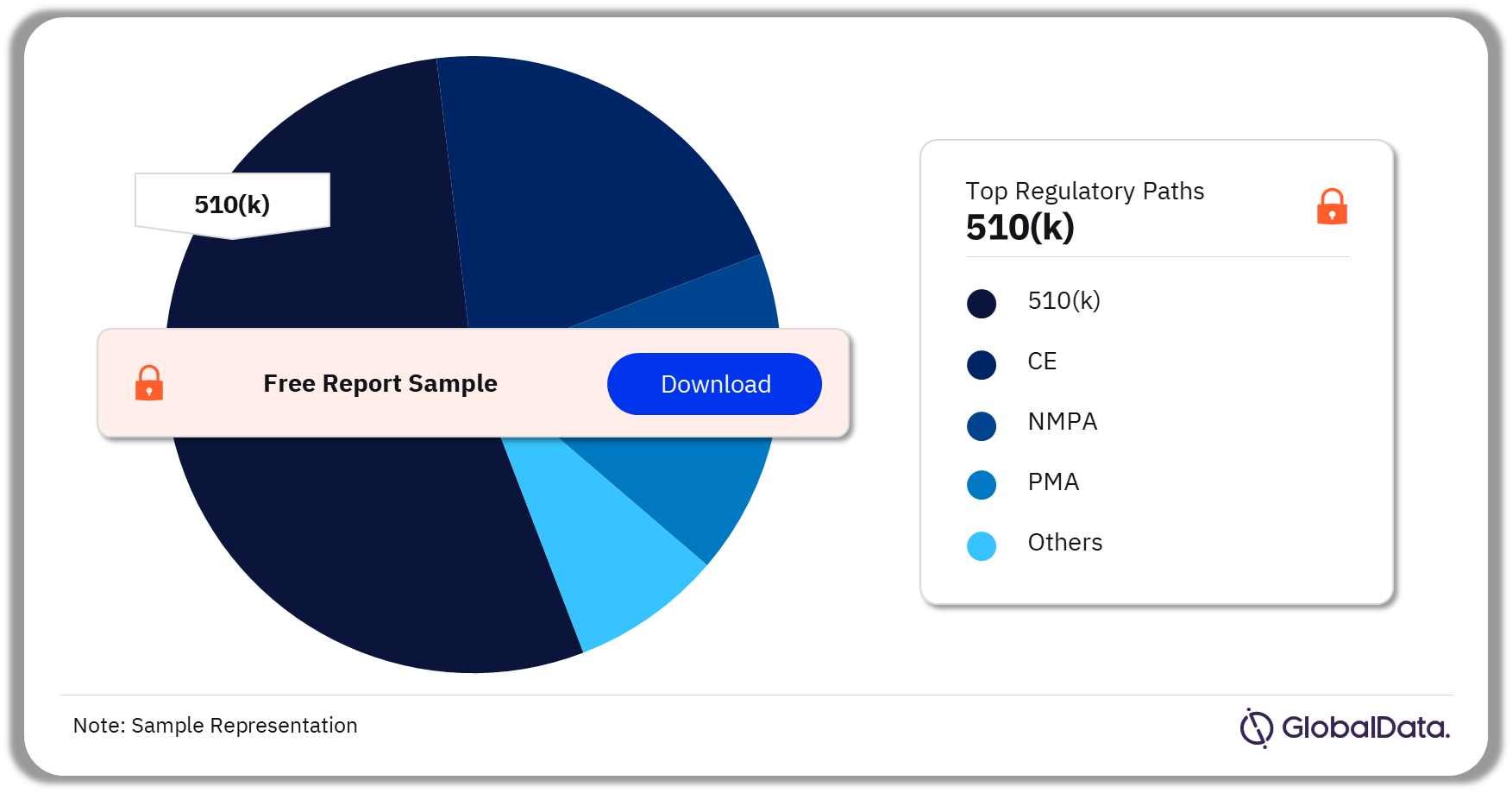
Spinal Fusion – Pipeline Products by Regulatory Path 31
Spinal Fusion – Pipeline Products by Estimated Approval Date 32
Spinal Fusion – Ongoing Clinical Trials 33
Spinal Fusion Companies – Pipeline Products by Stage of Development 34
Spinal Fusion – Pipeline Products by Stage of Development 39
Acuitive Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 44
Citrefuse – Product Status 44
Citrefuse – Product Description 44
Allegra Medical Technologies Ltd Pipeline Products & Ongoing Clinical Trials Overview 45
Sr-HT-Gahnite Spinal Cage Device – Product Status 45
Sr-HT-Gahnite Spinal Cage Device – Product Description 45
ALM Ortho Inc Pipeline Products & Ongoing Clinical Trials Overview 46
Lateral Intervertebral Fusion Cage – Product Status 46
Lateral Intervertebral Fusion Cage – Product Description 46
SI Joint Fusion Device – Product Status 47
SI Joint Fusion Device – Product Description 47
Alphatec Holdings Inc Pipeline Products & Ongoing Clinical Trials Overview 48
BioScorp – Product Status 48
BioScorp – Product Description 49
Helifuse – Product Status 49
Helifuse – Product Description 49
OsseoFix Next Generation Implant – Product Status 50
OsseoFix Next Generation Implant – Product Description 50
OsseoSleeve – Product Status 50
OsseoSleeve – Product Description 51
PCB – Product Status 51
PCB – Product Description 51
Samarys RF – Product Status 52
Samarys RF – Product Description 52
AMB Surgical II LLC Pipeline Products & Ongoing Clinical Trials Overview 53
FLYTE SPINE – Product Status 53
FLYTE SPINE – Product Description 53
APTO Orthopaedics Pipeline Products & Ongoing Clinical Trials Overview 54
Pediatric Spinal Fixation System – Product Status 54
Pediatric Spinal Fixation System – Product Description 54
Aurora Spine Corp Pipeline Products & Ongoing Clinical Trials Overview 55
Restore Kyphoplasty System – Product Status 55
Restore Kyphoplasty System – Product Description 55
SiLO-FX Fusion System – Product Status 56
SiLO-FX Fusion System – Product Description 56
VOX 2.0 – Product Status 56
VOX 2.0 – Product Description 57
Biedermann Motech GmbH & Co KG Pipeline Products & Ongoing Clinical Trials Overview 58
Mesh – Product Status 58
Mesh – Product Description 58
Bio2 Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 59
Vitrium – Cervical Interbody Fusion Device – Product Status 59
Vitrium – Cervical Interbody Fusion Device – Product Description 59
Vitrium – Lumbar Interbody Fusion Device – Product Status 60
Vitrium – Lumbar Interbody Fusion Device – Product Description 60
Bioretec Ltd Pipeline Products & Ongoing Clinical Trials Overview 61
RemeOs Spinal Cage – Product Status 61
RemeOs Spinal Cage – Product Description 61
Spinal Plate – Spinal Osteosynthesis – Product Status 62
Spinal Plate – Spinal Osteosynthesis – Product Description 62
Butterfly Spine, LLC Pipeline Products & Ongoing Clinical Trials Overview 63
Interspinous Dynamic Fusion Device – Product Status 63
Interspinous Dynamic Fusion Device – Product Description 63
Camber Spine Technologies LLC Pipeline Products & Ongoing Clinical Trials Overview 64
SPIRA – Expandable Transforaminal Lumbar Interbody – Product Status 64
SPIRA – Expandable Transforaminal Lumbar Interbody – Product Description 64
SPIRA – Sacroiliac – Product Status 65
SPIRA – Sacroiliac – Product Description 65
SPIRA Posterior Lumbar – Product Status 65
SPIRA Posterior Lumbar – Product Description 66
Carlsmed Inc Pipeline Products & Ongoing Clinical Trials Overview 67
Aprevo Cervical Spine Interbody Fusion Device – Product Status 67
Aprevo Cervical Spine Interbody Fusion Device – Product Description 67
Choice Spine LLC Pipeline Products & Ongoing Clinical Trials Overview 68
Expandable PEEK Interbody Cage – Product Status 68
Expandable PEEK Interbody Cage – Product Description 68
Next Generation Gibralt Spine System – Product Status 69
Next Generation Gibralt Spine System – Product Description 69
Next Generation Proliant Spine System – Product Status 69
Next Generation Proliant Spine System – Product Description 69
Clariance Inc Pipeline Products & Ongoing Clinical Trials Overview 70
Platform Access – Product Status 70
Platform Access – Product Description 70
Clariance SAS Pipeline Products & Ongoing Clinical Trials Overview 71
Erisma Deformity – Product Status 71
Erisma Deformity – Product Description 71
Clariance SAS – Ongoing Clinical Trials Overview 72
Erisma Deformity – Clariance Registry of ERISMA and Idys Devices 73
Corelink LLC Pipeline Products & Ongoing Clinical Trials Overview 74
3D Printed Cervical Titanium Interbody System – Product Status 74
3D Printed Cervical Titanium Interbody System – Product Description 74
3D Printed Lumbar Titanium Interbody System – Product Status 75
3D Printed Lumbar Titanium Interbody System – Product Description 75
Cervical Plate System – Product Status 75
Cervical Plate System – Product Description 75
Stand Alone Cervical Cage System – Product Status 76
Stand Alone Cervical Cage System – Product Description 76
Universal Pedicle Screw System – Product Status 76
Universal Pedicle Screw System – Product Description 76
Cortical Concepts (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 77
Cortical Anchor – Product Status 77
Cortical Anchor – Product Description 77
CTL Amedica Corp Pipeline Products & Ongoing Clinical Trials Overview 78
Sneak Peak – Product Status 78
Sneak Peak – Product Description 78
Curiteva Inc Pipeline Products & Ongoing Clinical Trials Overview 79
Inspire PLIF Interbody Implant – Product Status 79
Inspire PLIF Interbody Implant – Product Description 79
Inspire Standalone ALIF Interbody Implant – Product Status 80
Inspire Standalone ALIF Interbody Implant – Product Description 80
Inspire Static ALIF Interbody Implant – Product Status 80
Inspire Static ALIF Interbody Implant – Product Description 81
Inspire TLIF Interbody Implant – Product Status 81
Inspire TLIF Interbody Implant – Product Description 81
Cutting Edge Spine LLC Pipeline Products & Ongoing Clinical Trials Overview 82
EVOL HA-ALIF – Product Status 82
EVOL HA-ALIF – Product Description 82
Ventral Cervical Plate – Product Status 83
Ventral Cervical Plate – Product Description 83
DePuy Synthes Inc Pipeline Products & Ongoing Clinical Trials Overview 84
Expandable Cage – Spine – Product Status 84
Expandable Cage – Spine – Product Description 84
Expedium FAS 2 – Product Status 85
Expedium FAS 2 – Product Description 85
DSM Biomedical BV Pipeline Products & Ongoing Clinical Trials Overview 86
Graft Containment Device – Product Status 86
Graft Containment Device – Product Description 86
Porous Resorbable Interbody Spacer – Product Status 87
Porous Resorbable Interbody Spacer – Product Description 87
Spine Inter-Body Fusion Cage – Product Status 87
Spine Inter-Body Fusion Cage – Product Description 88
Structural Spine Implant – Product Status 88
Structural Spine Implant – Product Description 88
DSM Dyneema BV Pipeline Products & Ongoing Clinical Trials Overview 89
Dyneema Purity – Scoliosis – Product Status 89
Dyneema Purity – Scoliosis – Product Description 89
Eden Spine LLC (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 90
PERFX-1 – Product Status 90
PERFX-1 – Product Description 90
EDGe Surgical Inc Pipeline Products & Ongoing Clinical Trials Overview 91
Next Gen Spine Awl-In-One Tap System – Product Status 91
Next Gen Spine Awl-In-One Tap System – Product Description 91
EIT Emerging Implant Technologies GmbH Pipeline Products & Ongoing Clinical Trials Overview 92
Fully Printed Lateral Expanding Cage – Product Status 92
Fully Printed Lateral Expanding Cage – Product Description 92
Eminent Spine LLC Pipeline Products & Ongoing Clinical Trials Overview 93
Anaconda – Product Status 93
Anaconda – Product Description 93
King Snake – Product Status 94
King Snake – Product Description 94
Queen Snake – Product Status 94
Queen Snake – Product Description 95
ErgoSpine Pipeline Products & Ongoing Clinical Trials Overview 96
ErgoFuse – Product Status 96
ErgoFuse – Product Description 96
Evoke Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 97
eHeal – Product Status 97
eHeal – Product Description 97
FacetMed Ltd Pipeline Products & Ongoing Clinical Trials Overview 98
DualAxis – Product Status 98
DualAxis – Product Description 98
PediWing Spinal Fixation System – Product Status 99
PediWing Spinal Fixation System – Product Description 99
Fuse Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 100
Cervical Spinal Interbody Device – Product Status 100
Cervical Spinal Interbody Device – Product Description 100
Lumbar Spinal Interbody Device – Product Status 101
Lumbar Spinal Interbody Device – Product Description 101
GS Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 102
AnyPlus Anterior Cervical Plate – Product Status 102
AnyPlus Anterior Cervical Plate – Product Description 102
AnyPlus Posterior Cervical OCT Plate And Screw System – Product Status 103
AnyPlus Posterior Cervical OCT Plate And Screw System – Product Description 103
Chrome Cobalt Rod System – Product Status 103
Chrome Cobalt Rod System – Product Description 104
H. Lee Moffitt Cancer Center & Research Institute Inc Pipeline Products & Ongoing Clinical Trials Overview 105
Laterally Expanding Cage – Product Status 105
Laterally Expanding Cage – Product Description 105
Transdiscal Screw System – Product Status 106
Transdiscal Screw System – Product Description 106
HAPPE Spine LLC Pipeline Products & Ongoing Clinical Trials Overview 107
Lumbar PEEK Interbody Device – ALIF – Product Status 107
Lumbar PEEK Interbody Device – ALIF – Product Description 107
Lumbar PEEK Interbody Device – TLIF – Product Status 108
Lumbar PEEK Interbody Device – TLIF – Product Description 108
HD LifeSciences LLC Pipeline Products & Ongoing Clinical Trials Overview 109
Hive Cervical – Product Status 109
Hive Cervical – Product Description 109
Hospital for Special Surgery Pipeline Products & Ongoing Clinical Trials Overview 110
Percutaneous Graft Delivery System – Product Status 110
Percutaneous Graft Delivery System – Product Description 110
Implanet SA Pipeline Products & Ongoing Clinical Trials Overview 111
Hybrid Fixation System – Product Status 111
Hybrid Fixation System – Product Description 112
ISS-JAZZ Screw System – Product Status 112
ISS-JAZZ Screw System – Product Description 112
Jazz Cap System – Product Status 113
Jazz Cap System – Product Description 113
JAZZ Evo – Product Status 113
JAZZ Evo – Product Description 113
Jazz Lock – Product Status 114
Jazz Lock – Product Description 114
JAZZ Passer Band – Product Status 114
JAZZ Passer Band – Product Description 115
JAZZ Standalone Implant – Product Status 115
JAZZ Standalone Implant – Product Description 115
Spinal Deformity Device – Product Status 116
Spinal Deformity Device – Product Description 116
Inion Oy Pipeline Products & Ongoing Clinical Trials Overview 117
Lumbar Cage – Product Status 117
Lumbar Cage – Product Description 117
Innospina LLC Pipeline Products & Ongoing Clinical Trials Overview 118
Spinal Fusion Implant – Product Status 118
Spinal Fusion Implant – Product Description 118
Intelligent Implant Systems LLC Pipeline Products & Ongoing Clinical Trials Overview 119
LSIF – Product Status 119
LSIF – Product Description 119
Mind Lumbar-Thoracic Spinal System – Product Status 120
Mind Lumbar-Thoracic Spinal System – Product Description 120
Intelligent Implants Pipeline Products & Ongoing Clinical Trials Overview 121
SmartFuse System – Product Status 121
SmartFuse System – Product Description 121
Jemo Spine, LLC. Pipeline Products & Ongoing Clinical Trials Overview 122
Alta – Anterior Cervical Plate – Product Status 122
Alta – Anterior Cervical Plate – Product Description 122
Moab Interbody Spacer – Product Status 123
Moab Interbody Spacer – Product Description 123
joimax GmbH Pipeline Products & Ongoing Clinical Trials Overview 124
Disc Preparation Tool – LIF – Product Status 124
Disc Preparation Tool – LIF – Product Description 124
LESspine LLC Pipeline Products & Ongoing Clinical Trials Overview 125
Expanding P-Lift – Product Status 125
Expanding P-Lift – Product Description 125
PedFuse REly – Product Status 126
PedFuse REly – Product Description 126
SIJ-Fuse Rod – Product Status 126
SIJ-Fuse Rod – Product Description 127
VBR (Vertebral Body Replacement) Device – Product Status 127
VBR (Vertebral Body Replacement) Device – Product Description 127
X-Visibl – Product Status 128
X-Visibl – Product Description 128
Lifespans Ltd Pipeline Products & Ongoing Clinical Trials Overview 129
Lifespans Soft Spine Implant – Product Status 129
Lifespans Soft Spine Implant – Product Description 129
Maxigen Biotech Inc Pipeline Products & Ongoing Clinical Trials Overview 130
MaxiCage – Product Status 130
MaxiCage – Product Description 130
Mayo Clinic Pipeline Products & Ongoing Clinical Trials Overview 131
Pedicle Screw Washer – Product Status 131
Pedicle Screw Washer – Product Description 131
Medicrea Group Pipeline Products & Ongoing Clinical Trials Overview 132
Corpectomy Implant – Product Status 132
Corpectomy Implant – Product Description 132
Medos International Sarl Pipeline Products & Ongoing Clinical Trials Overview 133
TriALTIS Spine System – Product Status 133
TriALTIS Spine System – Product Description 133
Medtronic Plc Pipeline Products & Ongoing Clinical Trials Overview 134
Anterior L1/S1 Spine Plates – Product Status 134
Anterior L1/S1 Spine Plates – Product Description 134
Osteoporosis And Cannulated Pedicle Screw System – Product Status 135
Osteoporosis And Cannulated Pedicle Screw System – Product Description 135
Solera Fenestrated Screw – Product Status 135
Solera Fenestrated Screw – Product Description 135
U Spine II – Product Status 136
U Spine II – Product Description 136
Medyssey Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 137
ATHENA – Product Status 137
ATHENA – Product Description 137
Mekanika Inc Pipeline Products & Ongoing Clinical Trials Overview 138
Modulus System – Product Status 138
Modulus System – Product Description 138
Mercy Health Research Pipeline Products & Ongoing Clinical Trials Overview 139
Spinous Process Device – Product Status 139
Spinous Process Device – Product Description 139
Mesoblast Ltd Pipeline Products & Ongoing Clinical Trials Overview 140
NeoFuse – Product Status 140
NeoFuse – Product Description 140
MI4 Spine, LLC Pipeline Products & Ongoing Clinical Trials Overview 141
Cervical Plate – Product Status 141
Cervical Plate – Product Description 141
MIS Interspinous Process Spacer – Product Status 142
MIS Interspinous Process Spacer – Product Description 142
MIS Pedicle Screw – Product Status 142
MIS Pedicle Screw – Product Description 143
PLIF/TLIF System – Product Status 143
PLIF/TLIF System – Product Description 143
Michigan State University Pipeline Products & Ongoing Clinical Trials Overview 144
X-Plant Spinal Fixator – Product Status 144
X-Plant Spinal Fixator – Product Description 144
MicroPort Scientific Corp Pipeline Products & Ongoing Clinical Trials Overview 145
Spine Plate – Product Status 145
Spine Plate – Product Description 145
Spine Screw – Product Status 146
Spine Screw – Product Description 146
N8 Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 147
Coated Pedicle Screw – Product Status 147
Coated Pedicle Screw – Product Description 147
Nanovis Inc Pipeline Products & Ongoing Clinical Trials Overview 148
FortiSpan CrossLink – Product Status 148
FortiSpan CrossLink – Product Description 148
Nasseo, Inc. Pipeline Products & Ongoing Clinical Trials Overview 149
3D Printed Spinal Implant – Product Status 149
3D Printed Spinal Implant – Product Description 149
NEOS Surgery SL Pipeline Products & Ongoing Clinical Trials Overview 150
NEOS Pedicle Screw System – Product Status 150
NEOS Pedicle Screw System – Product Description 150
Neumedix Surgical Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 151
NeuMedix Expanding Cervical Canal Device – Product Status 151
NeuMedix Expanding Cervical Canal Device – Product Description 151
Nexilis AG Pipeline Products & Ongoing Clinical Trials Overview 152
Immediate Stabilization System – Product Status 152
Immediate Stabilization System – Product Description 152
Nexxt Spine LLC Pipeline Products & Ongoing Clinical Trials Overview 153
Bone Healing Spinal Implant – Product Status 153
Bone Healing Spinal Implant – Product Description 153
Integrated Plate System – Product Status 154
Integrated Plate System – Product Description 154
Novadip Biosciences SA Pipeline Products & Ongoing Clinical Trials Overview 155
NVD-001 – Product Status 155
NVD-001 – Product Description 155
Novum Medical Products, Inc. Pipeline Products & Ongoing Clinical Trials Overview 156
Anterior Lumbar Interbody Fusion Device – Product Status 156
Anterior Lumbar Interbody Fusion Device – Product Description 156
Lateral Lumbar Interbody Fusion Device – Product Status 157
Lateral Lumbar Interbody Fusion Device – Product Description 157
Nutech Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 158
NuFix II Facet Fusion Dowel – Product Status 158
NuFix II Facet Fusion Dowel – Product Description 158
NuVasive Inc Pipeline Products & Ongoing Clinical Trials Overview 159
Reline Complex – Product Status 159
Reline Complex – Product Description 159
Reline Edge – Product Status 160
Reline Edge – Product Description 160
Reline One – Product Status 160
Reline One – Product Description 160
Orchid Orthopedic Solutions LLC Pipeline Products & Ongoing Clinical Trials Overview 161
Thru-Fuze Device – Product Status 161
Thru-Fuze Device – Product Description 161
Orthobond Pipeline Products & Ongoing Clinical Trials Overview 162
Antimicrobial Pedicle Screw System – Product Status 162
Antimicrobial Pedicle Screw System – Product Description 162
Osseus Fusion Systems LLC Pipeline Products & Ongoing Clinical Trials Overview 163
Next Generation Aries – Cervical – Product Status 163
Next Generation Aries – Cervical – Product Description 163
Next Generation Aries – Expandable Lumbar Fusion Cage – Product Status 164
Next Generation Aries – Expandable Lumbar Fusion Cage – Product Description 164
OsteoGeneX Inc Pipeline Products & Ongoing Clinical Trials Overview 165
Spinal Fusion Device – Product Status 165
Spinal Fusion Device – Product Description 165
OsteoVantage Inc Pipeline Products & Ongoing Clinical Trials Overview 166
INDOS System – Product Status 166
INDOS System – Product Description 166
Ozop Surgical Corp (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 167
Adjustable ALIF Interbody Spinal Implant – Product Status 167
Adjustable ALIF Interbody Spinal Implant – Product Description 167
Pharmaco-Kinesis Corp Pipeline Products & Ongoing Clinical Trials Overview 168
Intelligent Interbody Fusion Device – Product Status 168
Intelligent Interbody Fusion Device – Product Description 168
Providence Medical Technology Inc Pipeline Products & Ongoing Clinical Trials Overview 169
Posterior Cervical Stabilization System – Product Status 169
Posterior Cervical Stabilization System – Product Description 169
Providence Medical Technology Inc – Ongoing Clinical Trials Overview 170
Posterior Cervical Stabilization System – Safety and Effectiveness of Posterior Cervical Stabilization System (PCSS) as an Adjunct to Posterior Cervical Fusion, When Used in Combination with ACDF in Treatment of Multi-level Cervical Degenerative Disease 171
RegeneEx Ltd. Pipeline Products & Ongoing Clinical Trials Overview 172
RegeneEx Minimally Invasive Device – Product Status 172
RegeneEx Minimally Invasive Device – Product Description 172
Resoimplant GmbH (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 173
Resospine – Product Status 173
Resospine – Product Description 173
RevBio Inc Pipeline Products & Ongoing Clinical Trials Overview 174
Tetranite – Spine Fusion – Product Status 174
Tetranite – Spine Fusion – Product Description 174
ReVivo Medical, LLC Pipeline Products & Ongoing Clinical Trials Overview 175
Cervical Spinal Fusion Plate – Product Status 175
Cervical Spinal Fusion Plate – Product Description 175
Sail Fusion LLC Pipeline Products & Ongoing Clinical Trials Overview 176
BowTie Sacroiliac Fusion System – Product Status 176
BowTie Sacroiliac Fusion System – Product Description 176
Sail Fusion LLC – Ongoing Clinical Trials Overview 177
BowTie Sacroiliac Fusion System – A Pilot Clinical Study to Assess the Effects of the Novel BowTie Sacroiliac Fusion System 178
SANUWAVE Health Inc Pipeline Products & Ongoing Clinical Trials Overview 179
orthoPACE – Spinal Fusion – Product Status 179
orthoPACE – Spinal Fusion – Product Description 179
SeaSpine, Inc. Pipeline Products & Ongoing Clinical Trials Overview 180
PROW – Degenerative Disc Disease – Product Status 180
PROW – Degenerative Disc Disease – Product Description 180
Signus Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 181
Axioma-C – Product Status 181
Axioma-C – Product Description 181
SINTX Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 182
3DP SN-PEEK Cervical Implant – Product Status 182
3DP SN-PEEK Cervical Implant – Product Description 182
Biologically Enhanced Spinal Fusion Device – Product Status 183
Biologically Enhanced Spinal Fusion Device – Product Description 183
Composite Silicon Nitride Interbody Device – Product Status 183
Composite Silicon Nitride Interbody Device – Product Description 183
FleX SN Porous Spinal Implant – Product Status 184
FleX SN Porous Spinal Implant – Product Description 184
Spinal Balance Inc. Pipeline Products & Ongoing Clinical Trials Overview 185
Bioactive Facet Screw System – Product Status 185
Bioactive Facet Screw System – Product Description 185
Interbody Cage – Product Status 186
Interbody Cage – Product Description 186
Spinal Elements Inc Pipeline Products & Ongoing Clinical Trials Overview 187
Zyre Facet Implant System – Product Status 187
Zyre Facet Implant System – Product Description 187
Spinal Resources Inc Pipeline Products & Ongoing Clinical Trials Overview 188
Swedge Pedicle Screw Fixation System – 4.75 mm – Product Status 188
Swedge Pedicle Screw Fixation System – 4.75 mm – Product Description 189
Swedge Pedicle Screw Fixation System – 5.0 mm – Product Status 189
Swedge Pedicle Screw Fixation System – 5.0 mm – Product Description 190
Swedge Posterior Cervical Stabilization System – Product Status 190
Swedge Posterior Cervical Stabilization System – Product Description 190
Spinal Simplicity LLC Pipeline Products & Ongoing Clinical Trials Overview 191
Scarab – Product Status 191
Scarab – Product Description 191
SpinalVu Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 192
X – Wave Cervical Interbody Spacer – Product Status 192
X – Wave Cervical Interbody Spacer – Product Description 192
X – Wave Lumbar Interbody Spacer – Product Status 193
X – Wave Lumbar Interbody Spacer – Product Description 193
Spine Wave Inc Pipeline Products & Ongoing Clinical Trials Overview 194
CapSure PS2 Spine System – Product Status 194
CapSure PS2 Spine System – Product Description 194
SpineGuard SA Pipeline Products & Ongoing Clinical Trials Overview 195
PediGuard Spine SmartScrew – Product Status 195
PediGuard Spine SmartScrew – Product Description 195
SpineSmith Holdings LLC Pipeline Products & Ongoing Clinical Trials Overview 196
Cervical Interbody Device – Product Status 196
Cervical Interbody Device – Product Description 196
Closed VisuALIF – Product Status 197
Closed VisuALIF – Product Description 197
Facet Fixation Device – Product Status 197
Facet Fixation Device – Product Description 198
Posterior Lumbar Interbody Fusion Device – Product Status 198
Posterior Lumbar Interbody Fusion Device – Product Description 198
SpineVision SA Pipeline Products & Ongoing Clinical Trials Overview 199
Flex+2 – Product Status 199
Flex+2 – Product Description 199
Sree Chitra Tirunal Institute for Medical Sciences & Technology Pipeline Products & Ongoing Clinical Trials Overview 200
Spinal Fixation System – Product Status 200
Spinal Fixation System – Product Description 200
Stryker Corp Pipeline Products & Ongoing Clinical Trials Overview 201
AlloCraft C-Ring – Product Status 201
AlloCraft C-Ring – Product Description 201
Bioactive Inter-body Device – Product Status 202
Bioactive Inter-body Device – Product Description 202
Spiral Radius 90D Spinal System – Product Status 202
Spiral Radius 90D Spinal System – Product Description 203
Taragenyx Ltd (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 204
TRGYX-001 – Product Status 204
TRGYX-001 – Product Description 204
Theradaptive Inc Pipeline Products & Ongoing Clinical Trials Overview 205
OsteoAdapt SP Spinal Fusion Implant – Product Status 205
OsteoAdapt SP Spinal Fusion Implant – Product Description 205
Theradaptive Inc – Ongoing Clinical Trials Overview 206
OsteoAdapt SP Spinal Fusion Implant – OsteoAdapt SP Feasibility Study A Prospective, Blinded, Controlled, Dose-Randomized Clinical Investigation of OsteoAdapt SP in Transforaminal Lumbar Interbody Fusion in Treatment of Symptomatic Degenerative Disease of The Lumbosacral Spine 207
Tissue Regeneration Systems, Inc. Pipeline Products & Ongoing Clinical Trials Overview 208
TRS Bioactive Implant – Spinal Fusion Surgery – Product Status 208
TRS Bioactive Implant – Spinal Fusion Surgery – Product Description 208
TranS1 Inc Pipeline Products & Ongoing Clinical Trials Overview 209
KeyLIF – Product Status 209
KeyLIF – Product Description 209
TruSpine Technologies Plc Pipeline Products & Ongoing Clinical Trials Overview 210
Cervi-LOK – Product Status 210
Cervi-LOK – Product Description 210
Faci-LOK – Product Status 211
Faci-LOK – Product Description 211
GRASP Laminoplasty Device – Product Status 211
GRASP Laminoplasty Device – Product Description 212
University of Colorado Pipeline Products & Ongoing Clinical Trials Overview 213
Laminoplasty Distractor System – Product Status 213
Laminoplasty Distractor System – Product Description 213
University of Colorado Anschutz Medical Campus Pipeline Products & Ongoing Clinical Trials Overview 214
Anterior Cervical Plating System – Product Status 214
Anterior Cervical Plating System – Product Description 214
University of Pittsburgh Pipeline Products & Ongoing Clinical Trials Overview 215
Self-Powered Smart Implant – Product Status 215
Self-Powered Smart Implant – Product Description 215
University of South Florida Pipeline Products & Ongoing Clinical Trials Overview 216
Asymmetric Disc Distracting Cage – Product Status 216
Asymmetric Disc Distracting Cage – Product Description 216
Cervical Plating System – Product Status 217
Cervical Plating System – Product Description 217
Occipito-Cervical Fixation Device – Product Status 217
Occipito-Cervical Fixation Device – Product Description 218
Polyaxial Fixation Device – Product Status 218
Polyaxial Fixation Device – Product Description 218
Polyaxial Screw Head – Product Status 219
Polyaxial Screw Head – Product Description 219
Vertebral Body Cage – Product Status 219
Vertebral Body Cage – Product Description 220
University of Toledo Pipeline Products & Ongoing Clinical Trials Overview 221
Anchoring Pedicle Screw – Product Status 221
Anchoring Pedicle Screw – Product Description 222
Bioactive Fusion Device – Product Status 222
Bioactive Fusion Device – Product Description 222
Minimally Invasive Intervertebral Cage – Product Status 223
Minimally Invasive Intervertebral Cage – Product Description 223
Pedicle Screw – Surgical Correction Of Spinal Deformities – Product Status 223
Pedicle Screw – Surgical Correction Of Spinal Deformities – Product Description 224
Retractable Pedicle Screw Assembly – Product Status 224
Retractable Pedicle Screw Assembly – Product Description 224
Self-Expanding Inter-Vertebral Cage – Product Status 225
Self-Expanding Inter-Vertebral Cage – Product Description 225
Shape Memory Alloy Pedicle Screw – Product Status 225
Shape Memory Alloy Pedicle Screw – Product Description 226
Spinal Deformity Correction Device – Product Status 226
Spinal Deformity Correction Device – Product Description 226
Stabilized Spinal Fixation Hook – Product Status 227
Stabilized Spinal Fixation Hook – Product Description 227
Transpedicular Accessing Device – Product Status 227
Transpedicular Accessing Device – Product Description 228
University of Utah Pipeline Products & Ongoing Clinical Trials Overview 229
Wasatch Loop System – Product Status 229
Wasatch Loop System – Product Description 229
University of Virginia Pipeline Products & Ongoing Clinical Trials Overview 230
Spinal Implant – Product Status 230
Spinal Implant – Product Description 230
VersaSpine LLC Pipeline Products & Ongoing Clinical Trials Overview 231
VersaSpine Dual-Ended Pedicle Screw System – Product Status 231
VersaSpine Dual-Ended Pedicle Screw System – Product Description 231
Vertech Inc Pipeline Products & Ongoing Clinical Trials Overview 232
Rotoplasty System – VCF – Product Status 232
Rotoplasty System – VCF – Product Description 232
Verticor (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 233
VClipse Cervical Plating System – Product Status 233
VClipse Cervical Plating System – Product Description 233
VGI Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 234
VerteLX – Product Status 234
VerteLX – Product Description 234
Vivonics Inc Pipeline Products & Ongoing Clinical Trials Overview 235
Midis Cage – Product Status 235
Midis Cage – Product Description 235
White Light Medical Pipeline Products & Ongoing Clinical Trials Overview 236
AccuSpine – Product Status 236
AccuSpine – Product Description 236
Xtremity & Spinal Solutions Ltd Pipeline Products & Ongoing Clinical Trials Overview 237
FILL AND FIX PEEK OPTIMA ANATOMIC Cervical Cage – Product Status 237
FILL AND FIX PEEK OPTIMA ANATOMIC Cervical Cage – Product Description 237
FILL AND FIX PEEK OPTIMA ANATOMIC Lumbar Cage – Product Status 238
FILL AND FIX PEEK OPTIMA ANATOMIC Lumbar Cage – Product Description 238
Yunyi (Beijing) Medical Device Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 239
3D Printed PEEK Artificial Vertebral Body – Product Status 239
3D Printed PEEK Artificial Vertebral Body – Product Description 239
Zyga Technology Inc Pipeline Products & Ongoing Clinical Trials Overview 240
Glyder Facet Resurfacing System – Product Status 240
Glyder Facet Resurfacing System – Product Description 240
ZygoFix Ltd Pipeline Products & Ongoing Clinical Trials Overview 241
ZLock Cervical Implant – Product Status 241
ZLock Cervical Implant – Product Description 241
ZLock Lumbar Spinal Fusion System – Product Status 242
ZLock Lumbar Spinal Fusion System – Product Description 242
ZLock SI Implant – Product Status 242
ZLock SI Implant – Product Description 243
ZygoFix Ltd – Ongoing Clinical Trials Overview 244
ZLock Lumbar Spinal Fusion System – A Clinical Study to Evaluate the Efficacy of zLOCK Spinal Facet Joint Fixation System in the Treatment of Severe Back and Leg Pain 245
ZLock Lumbar Spinal Fusion System – Safety and Efficacy Assessment of the zLock Facet Fusion System- A Pilot Study 245
ZLock Lumbar Spinal Fusion System – Safety and Efficacy Assessment of Using the zLOCK Facet Stabilization System 245
Glossary 386
![]()