Transdermal Drug Delivery – Pipeline Products by Stage of Development 31
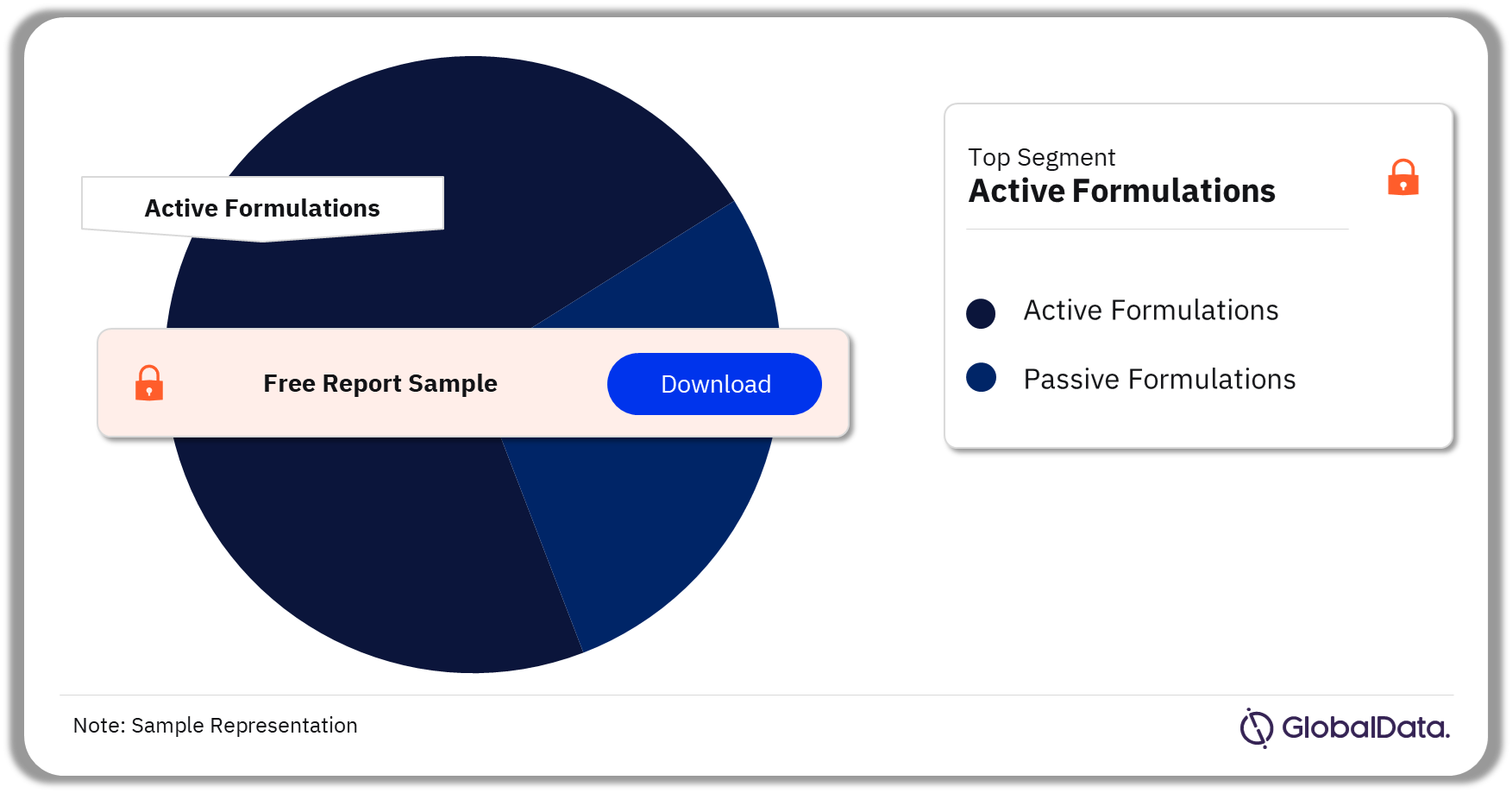
Transdermal Drug Delivery – Pipeline Products by Segment 32
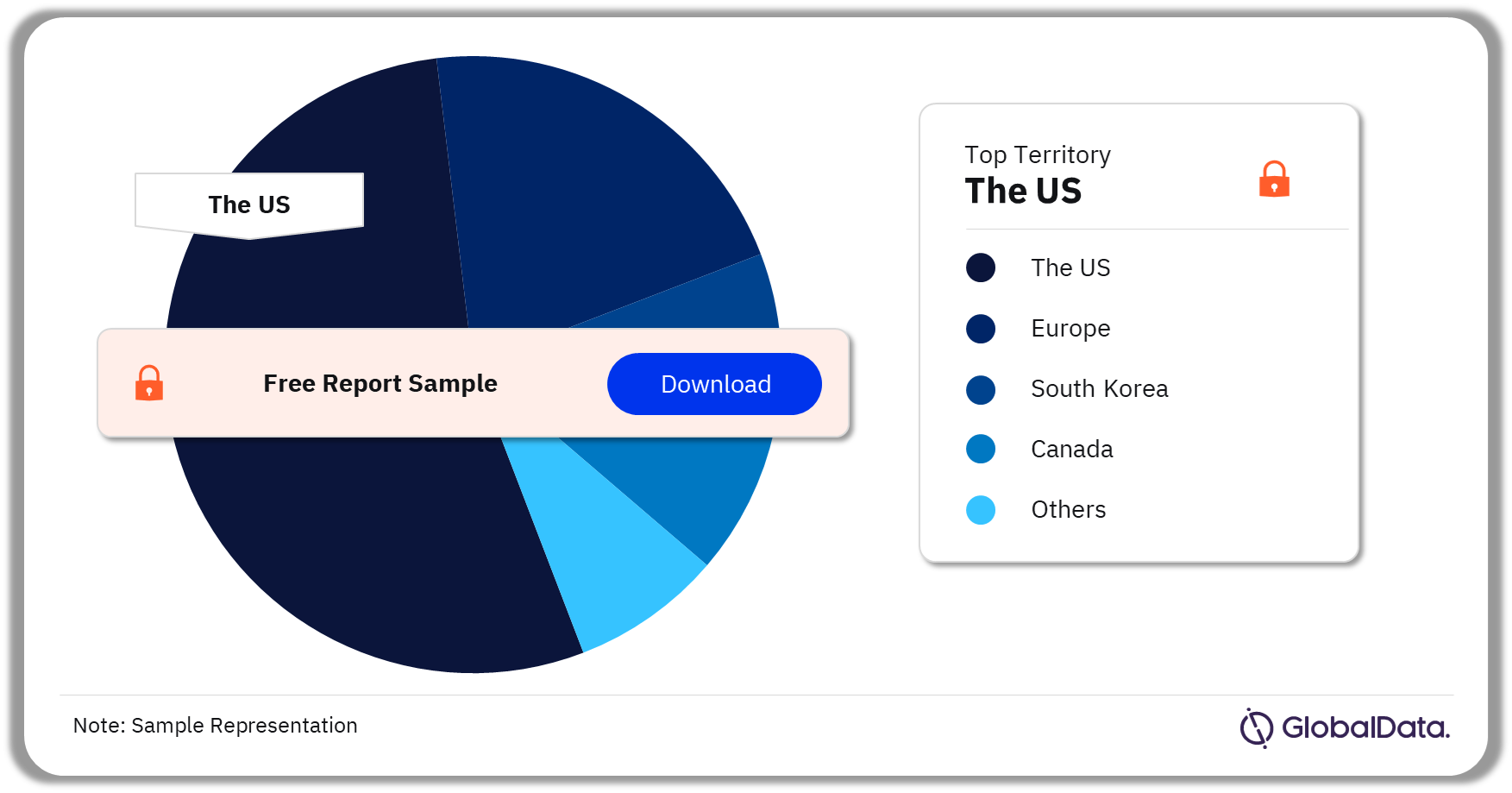
Transdermal Drug Delivery – Pipeline Products by Territory 33
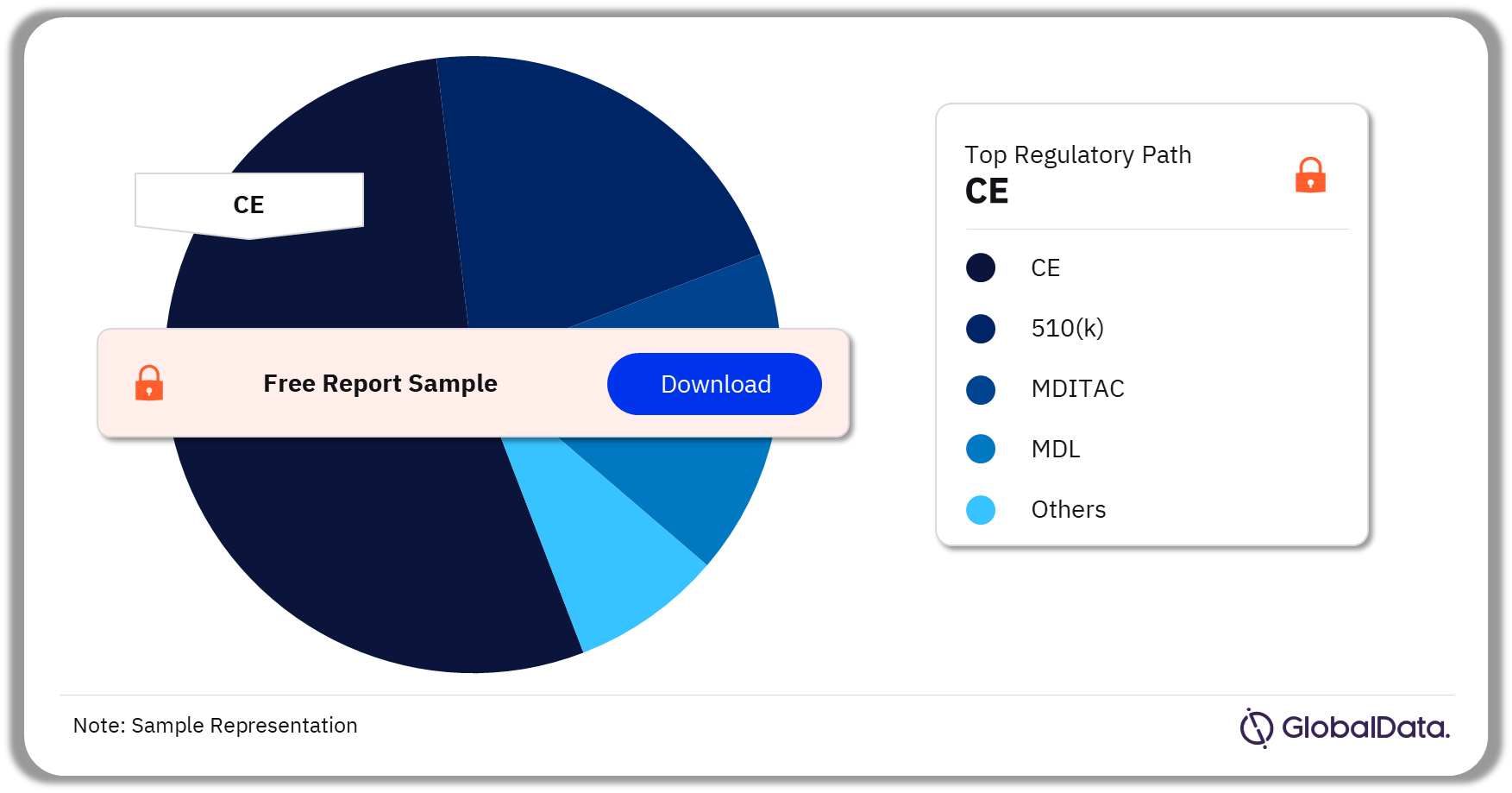
Transdermal Drug Delivery – Pipeline Products by Regulatory Path 35
Transdermal Drug Delivery – Pipeline Products by Estimated Approval Date 36
Transdermal Drug Delivery – Ongoing Clinical Trials 37
Transdermal Drug Delivery Companies – Pipeline Products by Stage of Development 38
Transdermal Drug Delivery – Pipeline Products by Stage of Development 45
3P Biotechnologies Inc Pipeline Products & Ongoing Clinical Trials Overview 53
PolyC – Product Status 53
PolyC – Product Description 53
4P Therapeutics LLC Pipeline Products & Ongoing Clinical Trials Overview 54
4P Apomorphine Transdermal Patch – Product Status 54
4P Apomorphine Transdermal Patch – Product Description 55
4P Chronic Pain Transdermal Patch – Product Status 55
4P Chronic Pain Transdermal Patch – Product Description 55
4P Clonidine Transdermal Patch – Product Status 55
4P Clonidine Transdermal Patch – Product Description 56
4P Diabetes Transdermal Patch – Product Status 56
4P Diabetes Transdermal Patch – Product Description 56
4P Lidocaine Transdermal Patch – Product Status 56
4P Lidocaine Transdermal Patch – Product Description 57
4P Migraine Transdermal Patch – Product Status 57
4P Migraine Transdermal Patch – Product Description 57
4P Scopolamine Transdermal Patch – Product Status 57
4P Scopolamine Transdermal Patch – Product Description 58
Aversa – Product Status 58
Aversa – Product Description 58
Aversa Buprenorphine Patch – Product Status 59
Aversa Buprenorphine Patch – Product Description 59
Aversa Methylphenidate Patch – Product Status 59
Aversa Methylphenidate Patch – Product Description 60
Transdermal Exenatide Patch – Product Status 60
Transdermal Exenatide Patch – Product Description 60
Transdermal FSH Patch – Product Status 61
Transdermal FSH Patch – Product Description 61
Abionic SA Pipeline Products & Ongoing Clinical Trials Overview 62
COVID-19 DNA Vaccine Microneedle Patch – Product Status 62
COVID-19 DNA Vaccine Microneedle Patch – Product Description 62
Actuated Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 63
GentleDispense System – Product Status 63
GentleDispense System – Product Description 63
Adynxx Inc Pipeline Products & Ongoing Clinical Trials Overview 64
Transdermal Postherpetic Neuralgia Pain Patch – Product Status 64
Transdermal Postherpetic Neuralgia Pain Patch – Product Description 64
Aequus Pharmaceuticals Inc Pipeline Products & Ongoing Clinical Trials Overview 65
AQS1301 – Product Status 65
AQS1301 – Product Description 66
AQS1302 – Product Status 66
AQS1302 – Product Description 66
AQS1303 – Product Status 67
AQS1303 – Product Description 67
AQS1304 – Product Status 67
AQS1304 – Product Description 68
AerMedX Ltd Pipeline Products & Ongoing Clinical Trials Overview 69
Deposition System – Product Status 69
Deposition System – Product Description 69
Agile Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 70
AG200-ER Patch – Product Status 70
AG200-ER Patch – Product Description 71
AG200-SP Patch – Product Status 71
AG200-SP Patch – Product Description 71
AG890 Patch – Product Status 72
AG890 Patch – Product Description 72
Allergan Ltd Pipeline Products & Ongoing Clinical Trials Overview 73
Nanotechnology-Based Transdermal Delivery System – Product Status 73
Nanotechnology-Based Transdermal Delivery System – Product Description 73
Norethindrone Transdermal Delivery System – Product Status 74
Norethindrone Transdermal Delivery System – Product Description 74
American Chemical Society Pipeline Products & Ongoing Clinical Trials Overview 75
COVID-19 Microneedle Patch – Product Status 75
COVID-19 Microneedle Patch – Product Description 75
AMW GmbH Pipeline Products & Ongoing Clinical Trials Overview 76
Anastrozole Transdermal Patch – Product Status 76
Anastrozole Transdermal Patch – Product Description 76
Amzell BV Pipeline Products & Ongoing Clinical Trials Overview 77
AMZ001 – Product Status 77
AMZ001 – Product Description 77
Apogee Technology Inc Pipeline Products & Ongoing Clinical Trials Overview 78
PyraDerm – Product Status 78
PyraDerm – Product Description 78
Asahi Kasei Pharma Corp Pipeline Products & Ongoing Clinical Trials Overview 79
Teribone Microneedle Patch System – Product Status 79
Teribone Microneedle Patch System – Product Description 79
ASCIL Biopharm Pipeline Products & Ongoing Clinical Trials Overview 80
Minimally Invasive Transdermal System – Product Status 80
Minimally Invasive Transdermal System – Product Description 80
Avecho Biotechnology Ltd Pipeline Products & Ongoing Clinical Trials Overview 81
TPM – Diclofenac Patch – Product Status 81
TPM – Diclofenac Patch – Product Description 81
TPM – Lidocaine Patch – Product Status 82
TPM – Lidocaine Patch – Product Description 82
TPM – Oxycodone Patch – Product Status 82
TPM – Oxycodone Patch – Product Description 83
TPM – Oxymorphone Patch – Product Status 83
TPM – Oxymorphone Patch – Product Description 83
Avro Life Sciences Pipeline Products & Ongoing Clinical Trials Overview 84
OmniDerm – Product Status 84
OmniDerm – Product Description 84
Remdesivir Transdermal Patch – Product Status 85
Remdesivir Transdermal Patch – Product Description 85
BioNxt Solutions Inc. Pipeline Products & Ongoing Clinical Trials Overview 86
Rotigotine Transdermal Patch – Product Status 86
Rotigotine Transdermal Patch – Product Description 86
BioNxt Solutions Inc. – Ongoing Clinical Trials Overview 87
Rotigotine Transdermal Patch – A Human Clinical Pilot Study to Assess the Relative Bioavailability, Skin Adhesion and Skin Tolerance of Rotigotine Transdermal Patch 88
Boryung Pharmaceutical Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 89
Donepezil Patch – Product Status 89
Donepezil Patch – Product Description 89
Burke Pharmaceuticals LLC Pipeline Products & Ongoing Clinical Trials Overview 90
BLU402 Hydrogel Patch Wart Kit – Product Status 90
BLU402 Hydrogel Patch Wart Kit – Product Description 90
Drug Delivery Patch – Product Status 91
Drug Delivery Patch – Product Description 91
Candesant Biomedical Inc Pipeline Products & Ongoing Clinical Trials Overview 92
Bi-Layer Patch – Facial Hyperhidrosis – Product Status 92
Bi-Layer Patch – Facial Hyperhidrosis – Product Description 92
Bi-Layer Patch – Palmar Hyperhidrosis – Product Status 93
Bi-Layer Patch – Palmar Hyperhidrosis – Product Description 93
Bi-Layer Patch – Plantar Hyperhidrosis – Product Status 93
Bi-Layer Patch – Plantar Hyperhidrosis – Product Description 94
Brella – Product Status 94
Brella – Product Description 94
Candesant Biomedical Inc – Ongoing Clinical Trials Overview 95
Brella – An Evaluation of the Safety and Effectiveness of the N-SWEAT Patch for the Treatment of Primary Axillary Hyperhidrosis or Excessive Axillary Sweating 96
Carnegie Mellon University Pipeline Products & Ongoing Clinical Trials Overview 97
Bioresorbable Drug Delivery Patch – Product Status 97
Bioresorbable Drug Delivery Patch – Product Description 97
Cassava Sciences Inc Pipeline Products & Ongoing Clinical Trials Overview 98
FENROCK – Product Status 98
FENROCK – Product Description 98
CB Scientific Inc Pipeline Products & Ongoing Clinical Trials Overview 99
Pain-Patch – Product Status 99
Pain-Patch – Product Description 99
Cerimon Pharmaceuticals, Inc. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 100
Topical Diclofenac Patch – Product Status 100
Topical Diclofenac Patch – Product Description 100
Chrono Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 101
Biologically-Timed Drug Delivery Device – Product Status 101
Biologically-Timed Drug Delivery Device – Product Description 101
Cipla Ltd Pipeline Products & Ongoing Clinical Trials Overview 102
Tizanidine Transdermal Patch – Product Status 102
Tizanidine Transdermal Patch – Product Description 102
Clexio Biosciences Ltd Pipeline Products & Ongoing Clinical Trials Overview 103
CLE-200 – Product Status 103
CLE-200 – Product Description 103
Corium Inc Pipeline Products & Ongoing Clinical Trials Overview 104
Corplex Memantine Transdermal System – Product Status 104
Corplex Memantine Transdermal System – Product Description 104
Corplex Ropinirole Transdermal System – Product Status 105
Corplex Ropinirole Transdermal System – Product Description 105
MicroCor hPTH(1-34) Transdermal System – Product Status 105
MicroCor hPTH(1-34) Transdermal System – Product Description 106
MicroCor Zolmitriptan Transdermal System – Product Status 106
MicroCor Zolmitriptan Transdermal System – Product Description 106
MicroJet – Product Status 107
MicroJet – Product Description 107
Daewon Pharmaceutical Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 108
DW-1022 – Product Status 108
DW-1022 – Product Description 108
Daewon Pharmaceutical Co Ltd – Ongoing Clinical Trials Overview 109
DW-1022 – A Study To Assess the Effects of the DW-1022 for Obesity Treatment 110
Daewoong Pharmaceutical Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 111
Donepezil Patch – Product Status 111
Donepezil Patch – Product Description 111
DBV Technologies SA Pipeline Products & Ongoing Clinical Trials Overview 112
Viaskin Milk – Product Status 112
Viaskin Milk – Product Description 113
Viaskin Peanut Allergy Patch – 1-3 Years – Product Status 113
Viaskin Peanut Allergy Patch – 1-3 Years – Product Description 113
Viaskin Peanut Allergy Patch – 4-11 Years – Product Status 114
Viaskin Peanut Allergy Patch – 4-11 Years – Product Description 114
Viaskin Peanut Allergy Patch – Adolescents and Adults – Product Status 114
Viaskin Peanut Allergy Patch – Adolescents and Adults – Product Description 115
Viaskin rPT – Product Status 115
Viaskin rPT – Product Description 115
DBV Technologies SA – Ongoing Clinical Trials Overview 116
Viaskin Peanut Allergy Patch – 1-3 Years – A Double-blind, Placebo-controlled, Randomized Phase III Trial to Assess the Safety and Efficacy of Viaskin Peanut in Peanut-allergic Young Children 1-3 Years of Age 117
Viaskin Peanut Allergy Patch – 1-3 Years – Open-label Follow-up Study of the PEPITES Study to Evaluate the Long-term Efficacy and Safety of Viaskin Peanut 117
Viaskin Peanut Allergy Patch – 4-11 Years – A Phase 3, Double-blind, Placebo-controlled, Randomized Study to Assess the Efficacy and Safety of Epicutaneous Immunotherapy With DBV712 250 µg in 4-7-year-old Children With Peanut Allergy (VITESSE) 118
Viaskin Peanut Allergy Patch – 4-11 Years – Viaskin Peanut Immunotherapy Trial to Evaluate Safety, Simplicity and Efficacy 118
DD Therapeutics LLC (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 119
Transdermal Delivery System – Product Status 119
Transdermal Delivery System – Product Description 119
Delpor Inc Pipeline Products & Ongoing Clinical Trials Overview 120
Risperidone Subcutaneous Implant – Product Status 120
Risperidone Subcutaneous Implant – Product Description 120
Tizanidine Implant – Product Status 121
Tizanidine Implant – Product Description 121
Dermisonics Inc Pipeline Products & Ongoing Clinical Trials Overview 122
U-Strip – Product Status 122
U-Strip – Product Description 122
Dharma Therapeutics, Inc. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 123
Iontophoretic Drug Delivery Patch – Product Status 123
Iontophoretic Drug Delivery Patch – Product Description 123
Diomics Corp Pipeline Products & Ongoing Clinical Trials Overview 124
Diocheck Antibody Indicator – Product Status 124
Diocheck Antibody Indicator – Product Description 124
Dong-A ST Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 125
Donepezil Transdermal Patch – Product Status 125
Donepezil Transdermal Patch – Product Description 125
Drexel University Pipeline Products & Ongoing Clinical Trials Overview 126
Ultrasonically Assisted Transcutaneous Drug Delivery Device – Product Status 126
Ultrasonically Assisted Transcutaneous Drug Delivery Device – Product Description 126
DURECT Corp Pipeline Products & Ongoing Clinical Trials Overview 127
Eladur – Product Status 127
Eladur – Product Description 127
TRANSDUR – Sufentanil – Product Status 128
TRANSDUR – Sufentanil – Product Description 128
Echo Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 129
Prelude SkinPrep System – Product Status 129
Prelude SkinPrep System – Product Description 129
Emplicure AB Pipeline Products & Ongoing Clinical Trials Overview 130
Micro-Needle Patch – Product Status 130
Micro-Needle Patch – Product Description 130
Endo International Plc Pipeline Products & Ongoing Clinical Trials Overview 131
Rivastigmine Patch – Product Status 131
Rivastigmine Patch – Product Description 131
Endomimetics LLC Pipeline Products & Ongoing Clinical Trials Overview 132
AVF Gel – Product Status 132
AVF Gel – Product Description 132
Extend Biosciences Inc Pipeline Products & Ongoing Clinical Trials Overview 133
Non-Invasive Needle-Free Skin Patch – Product Status 133
Non-Invasive Needle-Free Skin Patch – Product Description 133
Fe3 Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 134
Fe3 Patch – Product Status 134
Fe3 Patch – Product Description 134
Futura Medical Plc Pipeline Products & Ongoing Clinical Trials Overview 135
Eroxon – Product Status 135
Eroxon – Product Description 135
MED3000 – Rx – Product Status 136
MED3000 – Rx – Product Description 136
Futura Medical Plc – Ongoing Clinical Trials Overview 137
MED3000 – Rx – MED3000 Topical Gel for On-Demand Treatment of Post-Radical Prostatectomy Erectile Dysfunction 138
Georgetown University Pipeline Products & Ongoing Clinical Trials Overview 139
Passive Transdermal Drug Delivery Patch – Parkinson’s Disease – Product Status 139
Passive Transdermal Drug Delivery Patch – Parkinson’s Disease – Product Description 139
Georgia Institute of Technology Pipeline Products & Ongoing Clinical Trials Overview 140
Micron – Scale Arcing Device – Product Status 140
Micron – Scale Arcing Device – Product Description 140
Microneedle Patch – Measles – Product Status 141
Microneedle Patch – Measles – Product Description 141
Microneedle Patch – Polio – Product Status 141
Microneedle Patch – Polio – Product Description 142
Microneedle Patch – Rubella – Product Status 142
Microneedle Patch – Rubella – Product Description 142
Georgia State University Pipeline Products & Ongoing Clinical Trials Overview 143
Microneedle Patch – Flu – Product Status 143
Microneedle Patch – Flu – Product Description 143
GlobeStar Therapeutics Corp Pipeline Products & Ongoing Clinical Trials Overview 144
Transdermal PGE-1 Patch – Product Status 144
Transdermal PGE-1 Patch – Product Description 144
Henley Ion LLC Pipeline Products & Ongoing Clinical Trials Overview 145
Anesthesia Delivery Dental Device – Product Status 145
Anesthesia Delivery Dental Device – Product Description 145
Hera Health Solutions Pipeline Products & Ongoing Clinical Trials Overview 146
Biodegradable Subcutaneous Buprenorphine Releasing Implant – Product Status 146
Biodegradable Subcutaneous Buprenorphine Releasing Implant – Product Description 146
Hisamitsu Pharmaceutical Co Inc Pipeline Products & Ongoing Clinical Trials Overview 147
HP-1030 Adhesive Skin Patch – Product Status 147
HP-1030 Adhesive Skin Patch – Product Description 147
HP-5000 Topical Patch – Product Status 148
HP-5000 Topical Patch – Product Description 148
HTU-520 Patch – Product Status 148
HTU-520 Patch – Product Description 149
I M Sechenov Moscow State Medical University Pipeline Products & Ongoing Clinical Trials Overview 150
Anti-Cancer Patch – Product Status 150
Anti-Cancer Patch – Product Description 150
Icure Pharmaceutical Inc Pipeline Products & Ongoing Clinical Trials Overview 151
Donepezil Dementia Patch – Product Status 151
Donepezil Dementia Patch – Product Description 152
Exenatide Patch – Product Status 152
Exenatide Patch – Product Description 152
Fentanyl Pain Relief Patch – Product Status 153
Fentanyl Pain Relief Patch – Product Description 153
Fomoterol Patch – Product Status 153
Fomoterol Patch – Product Description 154
Melatonin Patch – Product Status 154
Melatonin Patch – Product Description 154
Physostigmine Patch – Product Status 155
Physostigmine Patch – Product Description 155
Pramipexole Parkinson’s Patch – Product Status 155
Pramipexole Parkinson’s Patch – Product Description 156
Rotigotine Patch – Product Status 156
Rotigotine Patch – Product Description 156
Varenicline Patch – Product Status 157
Varenicline Patch – Product Description 157
ImmunoMatrix LLC Pipeline Products & Ongoing Clinical Trials Overview 158
Dermal Skin Patch Delivery System – Product Status 158
Dermal Skin Patch Delivery System – Product Description 158
ImmuPatch Pipeline Products & Ongoing Clinical Trials Overview 159
Coated ImmuPatch – Product Status 159
Coated ImmuPatch – Product Description 159
Dissolvable ImmuPatch – Product Status 159
Dissolvable ImmuPatch – Product Description 160
Indian Institute of Science Pipeline Products & Ongoing Clinical Trials Overview 161
Drug Delivery Patch – Product Status 161
Drug Delivery Patch – Product Description 161
Indian Institute of Technology Bombay Pipeline Products & Ongoing Clinical Trials Overview 162
Poly (n-Vinyl Caprolactam) Transdermal Drug Delivery System – Product Status 162
Poly (n-Vinyl Caprolactam) Transdermal Drug Delivery System – Product Description 162
Indian Institute of Technology Hyderabad Pipeline Products & Ongoing Clinical Trials Overview 163
Transdermal Mucoadhesive Patch – Product Status 163
Transdermal Mucoadhesive Patch – Product Description 163
InMed Pharmaceuticals Inc Pipeline Products & Ongoing Clinical Trials Overview 164
INM-755 – Drug Delivery Device – Product Status 164
INM-755 – Drug Delivery Device – Product Description 164
MiDROPS INM-088 Drug Delivery Device – Product Status 165
MiDROPS INM-088 Drug Delivery Device – Product Description 165
Institute of Materials Research & Engineering Pipeline Products & Ongoing Clinical Trials Overview 166
Skin Breaching Device – Product Status 166
Skin Breaching Device – Product Description 166
Intercell AG (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 167
Travelers Diarrhea Vaccine Patch – Product Status 167
Travelers Diarrhea Vaccine Patch – Product Description 167
Interstitial NS Pipeline Products & Ongoing Clinical Trials Overview 168
NanoMAP – Product Status 168
NanoMAP – Product Description 168
Isis Biopolymer Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 169
Gentle Touch – Product Status 169
Gentle Touch – Product Description 169
IsisIQ CLI – Product Status 169
IsisIQ CLI – Product Description 170
James J. Peters VA Medical Center Pipeline Products & Ongoing Clinical Trials Overview 171
Iontophoresis Patch – Product Status 171
Iontophoresis Patch – Product Description 171
Janisys Pipeline Products & Ongoing Clinical Trials Overview 172
Janisys Drug Delivery System – Product Status 172
Janisys Drug Delivery System – Product Description 172
Jiaxing Xinwen Biotechnology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 173
Transdermal Drug Delivery System – Neurology – Product Status 173
Transdermal Drug Delivery System – Neurology – Product Description 173
Transdermal Drug Delivery System – Pain – Product Status 174
Transdermal Drug Delivery System – Pain – Product Description 174
Jubilee Biotech Pipeline Products & Ongoing Clinical Trials Overview 175
JUBIwatch – Product Status 175
JUBIwatch – Product Description 175
Karolinska Institute Pipeline Products & Ongoing Clinical Trials Overview 176
Microneedle Patch – MRSA – Product Status 176
Microneedle Patch – MRSA – Product Description 176
Kindeva Drug Delivery LP Pipeline Products & Ongoing Clinical Trials Overview 177
Hollow Microstructured Transdermal System – Product Status 177
Hollow Microstructured Transdermal System – Product Description 177
Solid Microneedles Transdermal System – Product Status 178
Solid Microneedles Transdermal System – Product Description 178
KTH Royal Institute of Technology Pipeline Products & Ongoing Clinical Trials Overview 179
Microneedle Skin Patch – Product Status 179
Microneedle Skin Patch – Product Description 179
Leo Pharma AS Pipeline Products & Ongoing Clinical Trials Overview 180
Microneedle Patch – Psoriasis – Product Status 180
Microneedle Patch – Psoriasis – Product Description 180
Livzon Pharmaceutical Group Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 181
Asenapine TTS – Product Status 181
Asenapine TTS – Product Description 181
Lohmann Therapie-Systeme AG Pipeline Products & Ongoing Clinical Trials Overview 182
Intradermal Microneedle Patch – Product Status 182
Intradermal Microneedle Patch – Product Description 182
Smart Patch – Product Status 183
Smart Patch – Product Description 183
Luye Pharma Group Ltd Pipeline Products & Ongoing Clinical Trials Overview 184
Apleek – Product Status 184
Apleek – Product Description 184
Rivalif – Product Status 185
Rivalif – Product Description 185
Magnetar Medical Devices Ltd Pipeline Products & Ongoing Clinical Trials Overview 186
RATO Patch – Product Status 186
RATO Patch – Product Description 186
Massachusetts Institute of Technology Pipeline Products & Ongoing Clinical Trials Overview 187
Microneedle Patch – Melanoma – Product Status 187
Microneedle Patch – Melanoma – Product Description 187
Wearable Ultrasound Patch – Product Status 188
Wearable Ultrasound Patch – Product Description 188
Mayne Pharma Group Ltd Pipeline Products & Ongoing Clinical Trials Overview 189
Motion Sickness Transdermal Delivery System Patch – Product Status 189
Motion Sickness Transdermal Delivery System Patch – Product Description 189
Medherant Ltd Pipeline Products & Ongoing Clinical Trials Overview 190
Diclofenac TEPI Patch – Product Status 190
Diclofenac TEPI Patch – Product Description 190
Donepezil TEPI Patch – Product Status 191
Donepezil TEPI Patch – Product Description 191
Dronabinol TEPI Patch – Product Status 191
Dronabinol TEPI Patch – Product Description 192
Ibuprofen TEPI Patch – Product Status 192
Ibuprofen TEPI Patch – Product Description 192
Lidocaine TEPI Patch – Product Status 193
Lidocaine TEPI Patch – Product Description 193
Memantine TEPI Patch – Product Status 193
Memantine TEPI Patch – Product Description 194
Methyl Salicylate TEPI Patch – Product Status 194
Methyl Salicylate TEPI Patch – Product Description 194
MEDRx Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 195
Etoreat – Product Status 195
Etoreat – Product Description 196
Lydolyte – Product Status 196
Lydolyte – Product Description 196
MRX-1OXT Patch – Product Status 197
MRX-1OXT Patch – Product Description 197
MRX-4TZT Patch – Product Status 197
MRX-4TZT Patch – Product Description 198
MRX-7LAT Lidocaine Patch – Product Status 198
MRX-7LAT Lidocaine Patch – Product Description 198
MRX-7MLL – Product Status 199
MRX-7MLL – Product Description 199
MRX-9FLT Patch – Product Status 199
MRX-9FLT Patch – Product Description 200
MedRx, Inc. Pipeline Products & Ongoing Clinical Trials Overview 201
KENZAN Microneedle Array – Product Status 201
KENZAN Microneedle Array – Product Description 201
Microdermics Inc Pipeline Products & Ongoing Clinical Trials Overview 202
Microneedle Drug Delivery System – Product Status 202
Microneedle Drug Delivery System – Product Description 202
Micron Biomedical Inc Pipeline Products & Ongoing Clinical Trials Overview 203
Microneedle Patch – Contraception – Product Status 203
Microneedle Patch – Contraception – Product Description 203
Microneedle Patch – Virus Infection – Product Status 204
Microneedle Patch – Virus Infection – Product Description 204
Micropoint Technologies Pte Ltd Pipeline Products & Ongoing Clinical Trials Overview 205
Drug-Coated Micropoint Patch – Product Status 205
Drug-Coated Micropoint Patch – Product Description 205
Mineed Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 206
Detachable and Dissolvable Microneedle Patch – Product Status 206
Detachable and Dissolvable Microneedle Patch – Product Description 206
Mycrodose Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 207
Psilocin Transdermal Drug Delivery System – Product Status 207
Psilocin Transdermal Drug Delivery System – Product Description 207
Transdermal Drug Delivery System – LSD – Product Status 208
Transdermal Drug Delivery System – LSD – Product Description 208
MyLife Technologies Pipeline Products & Ongoing Clinical Trials Overview 209
Ceramic Vaccine Patch – Product Status 209
Ceramic Vaccine Patch – Product Description 209
Nanyang Technological University Pipeline Products & Ongoing Clinical Trials Overview 210
Skin Pressure Device – Product Status 210
Skin Pressure Device – Product Description 210
National Dental Centre Singapore Pipeline Products & Ongoing Clinical Trials Overview 211
MN Patch – Product Status 211
MN Patch – Product Description 211
National University of Singapore Pipeline Products & Ongoing Clinical Trials Overview 212
Microneedle Granule – Drug Delivery – Product Status 212
Microneedle Granule – Drug Delivery – Product Description 212
Microneedle Skin Patch – Product Status 213
Microneedle Skin Patch – Product Description 213
Nemaura Pharma Ltd Pipeline Products & Ongoing Clinical Trials Overview 214
Memspatch – Product Status 214
Memspatch – Product Description 215
Microneedle Drug Delivery Device – Product Status 215
Microneedle Drug Delivery Device – Product Description 215
Micropatch – Product Status 215
Micropatch – Product Description 216
Mycrolator – Product Status 216
Mycrolator – Product Description 216
Transdermal Patch – Alzheimer’s Disease – Product Status 217
Transdermal Patch – Alzheimer’s Disease – Product Description 217
Transdermal Patch – Buprenorphine – Product Status 217
Transdermal Patch – Buprenorphine – Product Description 217
Transdermal Patch – Diclofenac – Product Status 218
Transdermal Patch – Diclofenac – Product Description 218
Transdermal Patch – Hypertension – Product Status 218
Transdermal Patch – Hypertension – Product Description 218
Transdermal Patch – Nicotine – Product Status 219
Transdermal Patch – Nicotine – Product Description 219
Transdermal Patch – Parkinson’s Disease – Product Status 219
Transdermal Patch – Parkinson’s Disease – Product Description 219
Transdermal Patch – Rivastigmine – Product Status 220
Transdermal Patch – Rivastigmine – Product Description 220
NeuroDerm Ltd Pipeline Products & Ongoing Clinical Trials Overview 221
ND0601 Dermal Patch – Product Status 221
ND0601 Dermal Patch – Product Description 221
ND0611 Dermal Patch – Product Status 222
ND0611 Dermal Patch – Product Description 222
Nexgel Inc Pipeline Products & Ongoing Clinical Trials Overview 223
Diclofenac Hydrogel Patch – Product Status 223
Diclofenac Hydrogel Patch – Product Description 223
Nitto Denko Corp Pipeline Products & Ongoing Clinical Trials Overview 224
Transdermal Enoxaparin Sodium Patch – Product Status 224
Transdermal Enoxaparin Sodium Patch – Product Description 224
Transdermal Fentanyl Citrate Patch – Product Status 225
Transdermal Fentanyl Citrate Patch – Product Description 225
Transdermal Protein Patch – Product Status 225
Transdermal Protein Patch – Product Description 226
North Carolina State University Pipeline Products & Ongoing Clinical Trials Overview 227
Antibody Delivery Microneedle Patch – Product Status 227
Antibody Delivery Microneedle Patch – Product Description 227
Intraconazole Microneedle Patch – Product Status 228
Intraconazole Microneedle Patch – Product Description 228
Skin Patch – Melanoma – Product Status 228
Skin Patch – Melanoma – Product Description 228
Nova Graphene Ballistics Inc Pipeline Products & Ongoing Clinical Trials Overview 229
Anti-Addiction Patch – Product Status 229
Anti-Addiction Patch – Product Description 229
Noven Pharmaceuticals Inc Pipeline Products & Ongoing Clinical Trials Overview 230
Lidocaine Topical Patch – Product Status 230
Lidocaine Topical Patch – Product Description 230
NutriBand Inc Pipeline Products & Ongoing Clinical Trials Overview 231
Ciprofloxacin Transdermal Patch – Product Status 231
Ciprofloxacin Transdermal Patch – Product Description 231
TD00A1 – Transdermal Drug Delivery System – Product Status 232
TD00A1 – Transdermal Drug Delivery System – Product Description 232
TD00G1 – Transdermal Drug Delivery System – Product Status 232
TD00G1 – Transdermal Drug Delivery System – Product Description 233
TD0CD1/TD0CD2 – Transdermal Drug Delivery System – Product Status 233
TD0CD1/TD0CD2 – Transdermal Drug Delivery System – Product Description 233
TD0ID1 – Transdermal Drug Delivery System – Product Status 234
TD0ID1 – Transdermal Drug Delivery System – Product Description 234
TD0IJD – Transdermal Drug Delivery System – Product Status 234
TD0IJD – Transdermal Drug Delivery System – Product Description 234
Nuvo Pharmaceuticals Inc Pipeline Products & Ongoing Clinical Trials Overview 235
Alprazolam Patch – Product Status 235
Alprazolam Patch – Product Description 235
Ohio State University Pipeline Products & Ongoing Clinical Trials Overview 236
4-HPR Patch – Product Status 236
4-HPR Patch – Product Description 236
Transdermal Wound Dressing – Product Status 237
Transdermal Wound Dressing – Product Description 237
Osprey Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 238
Limb Recovery System – Product Status 238
Limb Recovery System – Product Description 238
Pantec Biosolutions AG Pipeline Products & Ongoing Clinical Trials Overview 239
P.L.E.A.S.E. Diclofenac/Lidocaine Device – Product Status 239
P.L.E.A.S.E. Diclofenac/Lidocaine Device – Product Description 239
P.L.E.A.S.E. IT Device – Product Status 240
P.L.E.A.S.E. IT Device – Product Description 240
P.L.E.A.S.E. Mobile – Product Status 240
P.L.E.A.S.E. Mobile – Product Description 241
P.L.E.A.S.E. Private – Product Status 241
P.L.E.A.S.E. Private – Product Description 241
P.L.E.A.S.E. RNAi – Product Status 241
P.L.E.A.S.E. RNAi – Product Description 242
PassPort Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 243
PassPort System – Product Status 243
PassPort System – Product Description 243
Pebble Inc Pipeline Products & Ongoing Clinical Trials Overview 244
Smart Drug Delivery System – Product Status 244
Smart Drug Delivery System – Product Description 244
PharmaTher Holdings Ltd Pipeline Products & Ongoing Clinical Trials Overview 245
DMT MN-Patch – Major Depressive Disorder – Product Status 245
DMT MN-Patch – Major Depressive Disorder – Product Description 245
MacroDose-MN Patch – Product Status 246
MacroDose-MN Patch – Product Description 246
MDMA MN Patch – Mental Disorders – Product Status 246
MDMA MN Patch – Mental Disorders – Product Description 247
MicroDose-MN Patch – Product Status 247
MicroDose-MN Patch – Product Description 247
PHARMAPATCH – Product Status 248
PHARMAPATCH – Product Description 248
Privo Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 249
PRV111 – Product Status 249
PRV111 – Product Description 249
Prometheon Pharma LLC Pipeline Products & Ongoing Clinical Trials Overview 250
HeatWave Patch – Product Status 250
HeatWave Patch – Product Description 250
TruePatch – Basal Insulin – Product Status 251
TruePatch – Basal Insulin – Product Description 251
TruePatch – Rapid-Acting (Bolus) Insulin – Product Status 251
TruePatch – Rapid-Acting (Bolus) Insulin – Product Description 252
Purdue University Pipeline Products & Ongoing Clinical Trials Overview 253
Flexible Elastomer Patch – Product Status 253
Flexible Elastomer Patch – Product Description 253
Microneedle Patch – Wound Oxygenation – Product Status 254
Microneedle Patch – Wound Oxygenation – Product Description 254
Pump For Drug-Delivery Patch – Product Status 254
Pump For Drug-Delivery Patch – Product Description 255
Quadmedicine Pipeline Products & Ongoing Clinical Trials Overview 256
Microneedle Drug Delivery Device – Obesity – Product Status 256
Microneedle Drug Delivery Device – Obesity – Product Description 256
Microneedle Drug Delivery Device – Osteoporosis – Product Status 257
Microneedle Drug Delivery Device – Osteoporosis – Product Description 257
QMM-P010 – Product Status 257
QMM-P010 – Product Description 258
QMV-P007 – Product Status 258
QMV-P007 – Product Description 258
QMV-P008 – Product Status 259
QMV-P008 – Product Description 259
Quoin Pharmaceuticals Inc Pipeline Products & Ongoing Clinical Trials Overview 260
QRX001 – Product Status 260
QRX001 – Product Description 260
QRX002 – Product Status 261
QRX002 – Product Description 261
Radius Health Inc Pipeline Products & Ongoing Clinical Trials Overview 262
Tymlos Patch – Product Status 262
Tymlos Patch – Product Description 262
Rescue Biomedical
![]()