Ultrasound Systems – Pipeline Products by Stage of Development 28
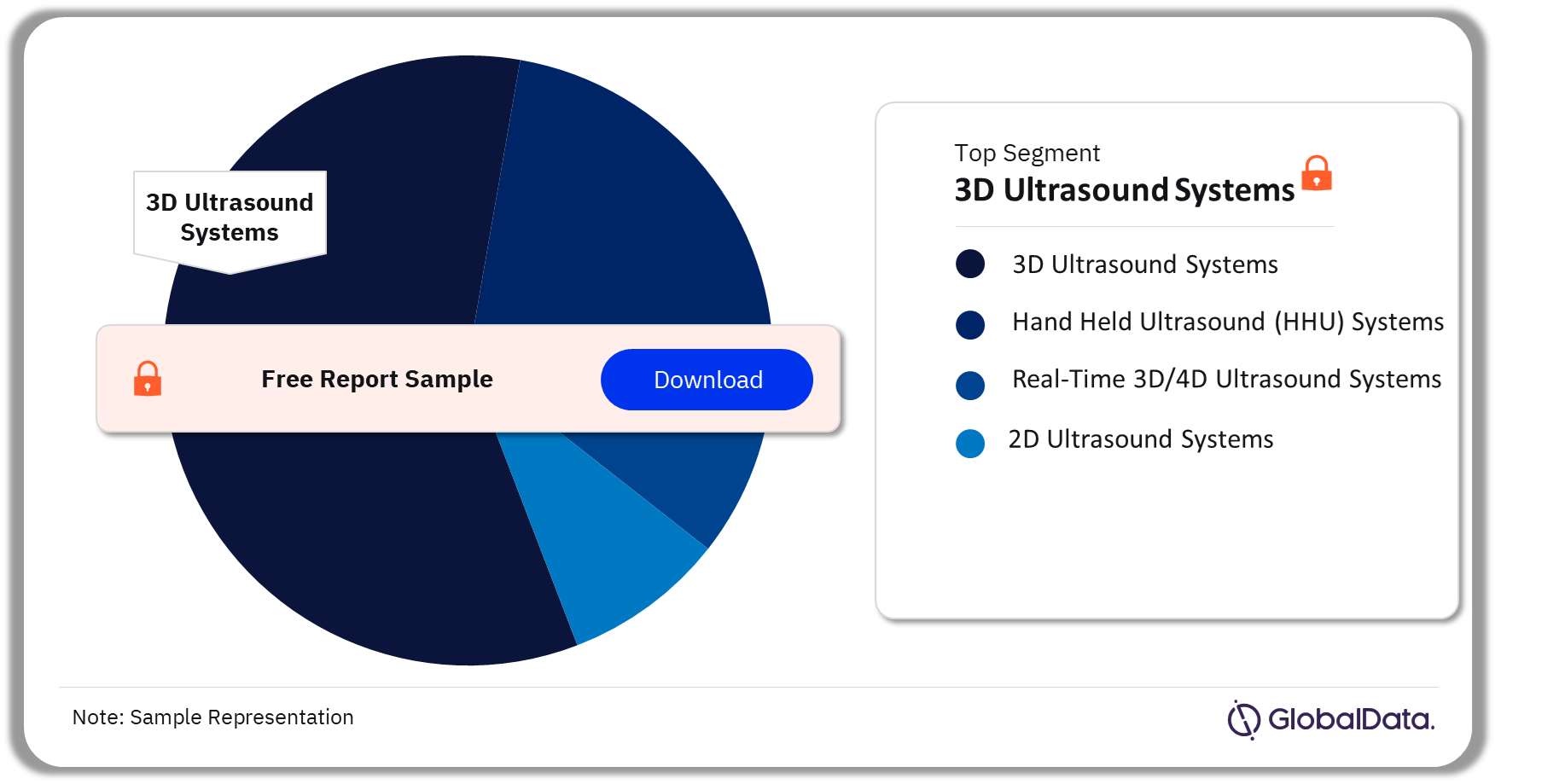
Ultrasound Systems – Pipeline Products by Segment 29
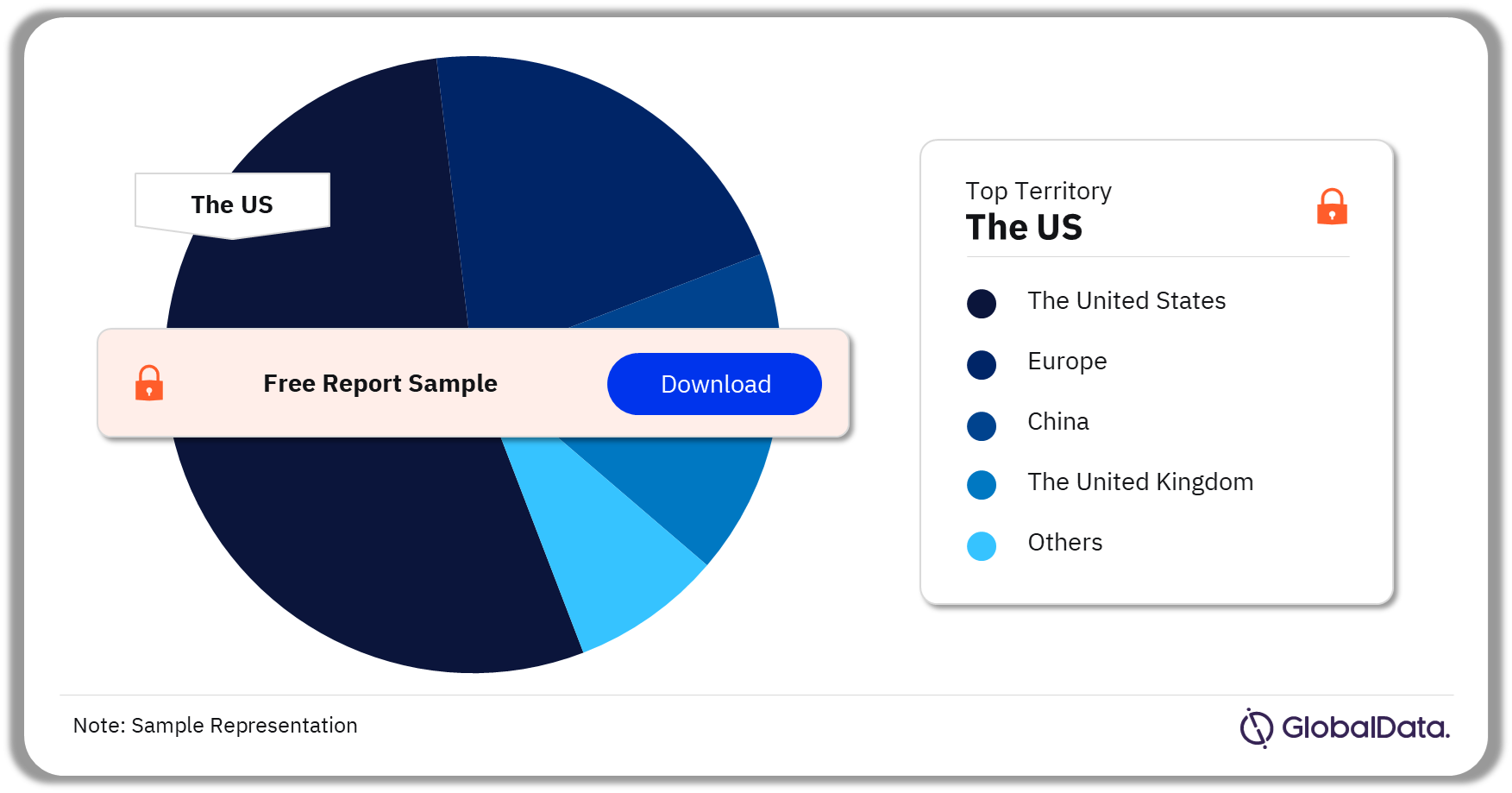
Ultrasound Systems – Pipeline Products by Territory 30
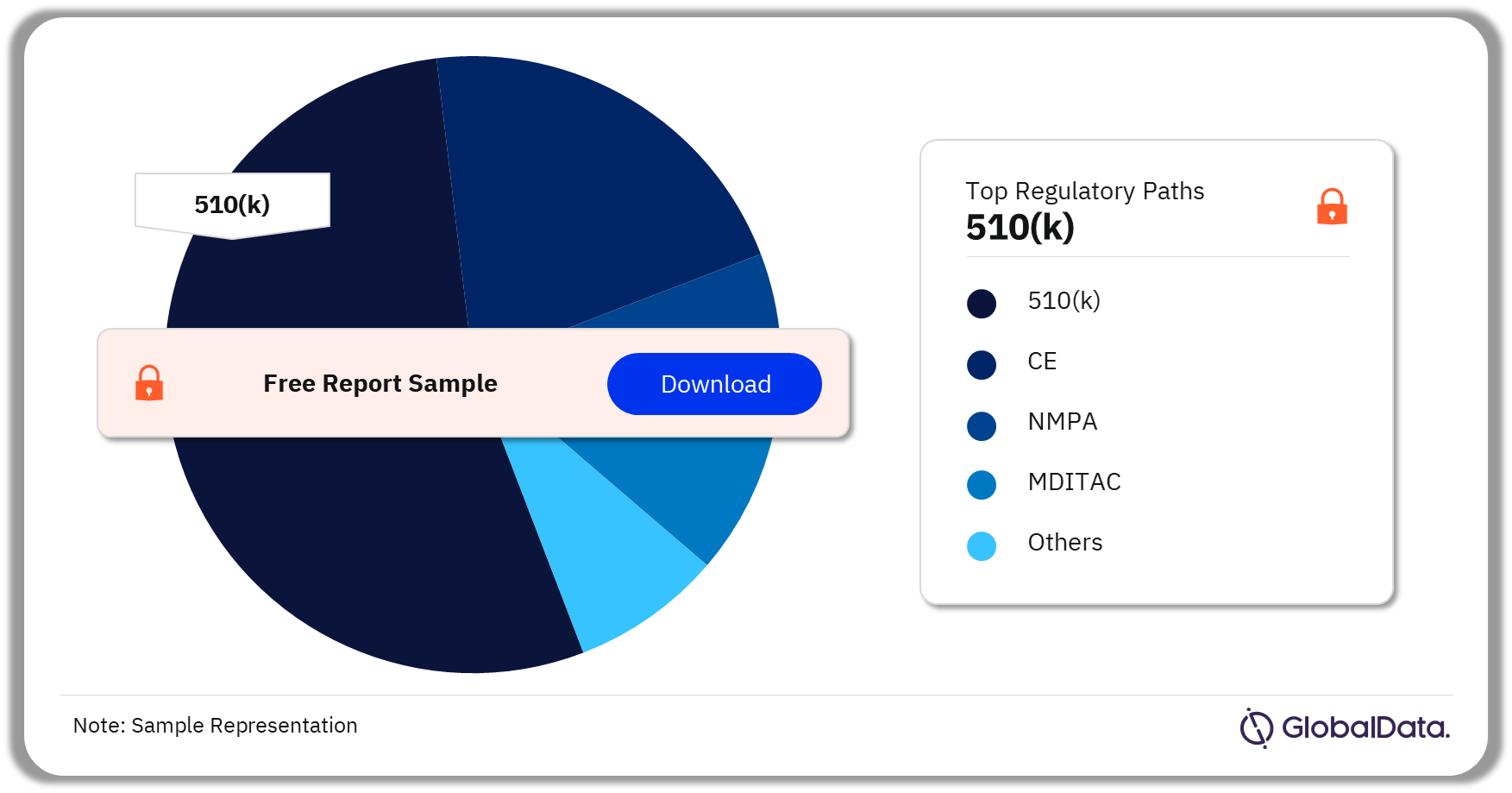
Ultrasound Systems – Pipeline Products by Regulatory Path 31
Ultrasound Systems – Pipeline Products by Estimated Approval Date 32
Ultrasound Systems – Ongoing Clinical Trials 33
Ultrasound Systems Companies – Pipeline Products by Stage of Development 34
Ultrasound Systems – Pipeline Products by Stage of Development 40
Acoustiic Inc Pipeline Products & Ongoing Clinical Trials Overview 46
AgilitUS – Product Status 46
AgilitUS – Product Description 46
Adenocyte LLC Pipeline Products & Ongoing Clinical Trials Overview 47
Linfu – Product Status 47
Linfu – Product Description 47
Adenocyte LLC – Ongoing Clinical Trials Overview 48
Linfu – The LINFU (A Noninvasive Method for Increasing Exfoliation of Pancreatic Ductal Cells Into Pancreatic Fluid) U.S. Registry for the Detection of PanIn-2, PanIn-3, and Early, Asymptomatic Pancreatic Ductal Adenocarcinoma (PDAC) 49
Advanced Medical Diagnostics, s.a. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 50
Breast HistoScanning – Product Status 50
Breast HistoScanning – Product Description 50
Ovarian HistoScanning – Product Status 51
Ovarian HistoScanning – Product Description 51
Thyroid HistoScanning – Product Status 51
Thyroid HistoScanning – Product Description 52
Advenio Tecnosys Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 53
Ultrasonography CAD – Product Status 53
Ultrasonography CAD – Product Description 53
Air Force Research Laboratory Pipeline Products & Ongoing Clinical Trials Overview 54
Flexible Ultrasound Patch – Product Status 54
Flexible Ultrasound Patch – Product Description 54
ALPINION Medical Systems Co., Ltd. Pipeline Products & Ongoing Clinical Trials Overview 55
VIFU 5000 – Breast Cancer – Product Status 55
VIFU 5000 – Breast Cancer – Product Description 55
VIFU 5000 – Pancreatic Cancer – Product Status 56
VIFU 5000 – Pancreatic Cancer – Product Description 56
VIFU 5000 – Prostate Cancer – Product Status 56
VIFU 5000 – Prostate Cancer – Product Description 57
VIFU 5000 – Uterine Cancer – Product Status 57
VIFU 5000 – Uterine Cancer – Product Description 57
Analogic Corp Pipeline Products & Ongoing Clinical Trials Overview 58
SonixTOUCH With Aegis Navigation System – Product Status 58
SonixTOUCH With Aegis Navigation System – Product Description 58
Transducer – Neonatal Imaging – Product Status 59
Transducer – Neonatal Imaging – Product Description 59
Angiogenesis Analytics BV Pipeline Products & Ongoing Clinical Trials Overview 60
PCaVision – Product Status 60
PCaVision – Product Description 60
Arbor Ultrasound Technologies, LLC (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 61
VF Doppler System – Product Status 61
VF Doppler System – Product Description 61
Bates Medical Systems LLC Pipeline Products & Ongoing Clinical Trials Overview 62
Automated Breast Ultrasound System – Product Status 62
Automated Breast Ultrasound System – Product Description 62
Becton Dickinson and Co Pipeline Products & Ongoing Clinical Trials Overview 63
Site-Rite 9 Ultrasound – Product Status 63
Site-Rite 9 Ultrasound – Product Description 63
Beijing East Whale Image Technology Co.,Ltd. Pipeline Products & Ongoing Clinical Trials Overview 64
Sigma P9 Echo – Product Status 64
Sigma P9 Echo – Product Description 64
BioSensics LLC Pipeline Products & Ongoing Clinical Trials Overview 65
Portable Ultrasound System – Product Status 65
Portable Ultrasound System – Product Description 65
BK Ultrasound Pipeline Products & Ongoing Clinical Trials Overview 66
UltraView 800 – Contrast Enhanced Ultrasound Imaging – Product Status 66
UltraView 800 – Contrast Enhanced Ultrasound Imaging – Product Description 66
UltraView 800 – HistoScanning – Product Status 67
UltraView 800 – HistoScanning – Product Description 67
Burl Concepts Inc Pipeline Products & Ongoing Clinical Trials Overview 68
Sonas – Product Status 68
Sonas – Product Description 68
Butterfly Network Inc Pipeline Products & Ongoing Clinical Trials Overview 69
Butterfly iQ+ Ultrasound System – Home Use – Product Status 69
Butterfly iQ+ Ultrasound System – Home Use – Product Description 69
Canon Medical Systems Corp Pipeline Products & Ongoing Clinical Trials Overview 70
Aplio i700 Diagnostic Ultrasound System – Prism Edition – Product Status 70
Aplio i700 Diagnostic Ultrasound System – Prism Edition – Product Description 70
Aplio i800 Diagnostic Ultrasound System – Prism Edition – Product Status 71
Aplio i800 Diagnostic Ultrasound System – Prism Edition – Product Description 71
Aplio i900 Diagnostic Ultrasound System – Prism Edition – Product Status 71
Aplio i900 Diagnostic Ultrasound System – Prism Edition – Product Description 72
Clarisond, Inc. Pipeline Products & Ongoing Clinical Trials Overview 73
Signal Processing System – Product Status 73
Signal Processing System – Product Description 73
Cleveland Clinic Lerner College of Medicine Pipeline Products & Ongoing Clinical Trials Overview 74
Diagnostic Imaging Device – Brain Disorders – Product Status 74
Diagnostic Imaging Device – Brain Disorders – Product Description 74
Daxsonics Ultrasound Pipeline Products & Ongoing Clinical Trials Overview 75
Compact Ultrasound Device – Product Status 75
Compact Ultrasound Device – Product Description 75
Department of Biomedical Engineering Columbia University Pipeline Products & Ongoing Clinical Trials Overview 76
Sono-Patch – Wearable Imaging Device – Product Status 76
Sono-Patch – Wearable Imaging Device – Product Description 76
Dr. Susan Love Research Foundation Pipeline Products & Ongoing Clinical Trials Overview 77
Ultrasound Device – Breast Cancer – Product Status 77
Ultrasound Device – Breast Cancer – Product Description 77
Duke University Pipeline Products & Ongoing Clinical Trials Overview 78
Acoustic Radiation Force Based Imaging – Product Status 78
Acoustic Radiation Force Based Imaging – Product Description 78
EchOpen Factory Pipeline Products & Ongoing Clinical Trials Overview 79
EchOpen Ultra-Portable Ultrasound – Product Status 79
EchOpen Ultra-Portable Ultrasound – Product Description 79
EchOpen Factory – Ongoing Clinical Trials Overview 80
EchOpen Ultra-Portable Ultrasound – Performance of the Echopen Probe in Its Clinical Use for Pregnancy Follow-up in Senegal 81
Edan Instruments Inc Pipeline Products & Ongoing Clinical Trials Overview 82
Virtuoso Compact Ultrasound System – Product Status 82
Virtuoso Compact Ultrasound System – Product Description 82
Emprenure Labs Pipeline Products & Ongoing Clinical Trials Overview 83
M-Ultra – Product Status 83
M-Ultra – Product Description 83
Endra Inc Pipeline Products & Ongoing Clinical Trials Overview 84
TAEUS FLIP – Product Status 84
TAEUS FLIP – Product Description 84
TAEUS System – Tissue Perfusion – Product Status 85
TAEUS System – Tissue Perfusion – Product Description 85
TAEUS System – Tissue Temperature – Product Status 85
TAEUS System – Tissue Temperature – Product Description 86
TAEUS System – Vascular Imaging – Product Status 86
TAEUS System – Vascular Imaging – Product Description 86
Endra Inc – Ongoing Clinical Trials Overview 87
TAEUS FLIP – A Clinical Study of ENDRA’s Thermo-acoustic Enhanced Ultrasound (TAEUS) System in Patients with Non-alcoholic Fatty Liver Disease 88
TAEUS FLIP – A Clinical Study to Evaluate the Performance of ENDRA’s Thermo-acoustic Enhanced Ultrasound (TAEUS) System in Patients with Non-alcoholic Fatty Liver Disease 88
TAEUS FLIP – Feasibility Study for Quantifying Fatty Liver Using Thermo-acoustic Imaging 88
TAEUS FLIP – Study to Evaluate the Efficacy of ENDRA’s Thermo Acoustic Enhanced Ultrasound (TAEUS) Device in Patients with Non-alcoholic Fatty Liver Disease 89
Esaote SpA Pipeline Products & Ongoing Clinical Trials Overview 90
MyLab A50 – Product Status 90
MyLab A50 – Product Description 90
MyLab A70 – Product Status 91
MyLab A70 – Product Description 91
EveryBaby Ltd Pipeline Products & Ongoing Clinical Trials Overview 92
Premature Birth Imaging Tool – Product Status 92
Premature Birth Imaging Tool – Product Description 92
EXACT Therapeutics AS Pipeline Products & Ongoing Clinical Trials Overview 93
Next Generation Ultrasound Transducer and Navigation System – ACT – Product Status 93
Next Generation Ultrasound Transducer and Navigation System – ACT – Product Description 93
eXo Imaging Inc Pipeline Products & Ongoing Clinical Trials Overview 94
Cello Ultrasound System – Product Status 94
Cello Ultrasound System – Product Description 94
Farus LLC Pipeline Products & Ongoing Clinical Trials Overview 95
FMUS Transducer Array – Product Status 95
FMUS Transducer Array – Product Description 95
Ultrasound System – Product Status 96
Ultrasound System – Product Description 96
Fraunhofer Institute for Biomedical Engineering Pipeline Products & Ongoing Clinical Trials Overview 97
Diphas Kombus – Product Status 97
Diphas Kombus – Product Description 97
Fraunhofer-Gesellschaft zur Forderung der Angewandten Forschung eV Pipeline Products & Ongoing Clinical Trials Overview 98
Multimodal Hybrid Ultrasound Imaging System – Product Status 98
Multimodal Hybrid Ultrasound Imaging System – Product Description 98
French National Institute of Health and Medical Research Pipeline Products & Ongoing Clinical Trials Overview 99
CMUT-Based Equipment With Ultrasound – Product Status 99
CMUT-Based Equipment With Ultrasound – Product Description 99
ElastoCardioScope – Product Status 100
ElastoCardioScope – Product Description 100
Fujifilm SonoSite Inc Pipeline Products & Ongoing Clinical Trials Overview 101
Cardiac Resuscitation Exam Type – Product Status 101
Cardiac Resuscitation Exam Type – Product Description 101
Sonosite LX Ultrasound System – Product Status 102
Sonosite LX Ultrasound System – Product Description 102
T8-3 Transoesophageal Transducer – Product Status 102
T8-3 Transoesophageal Transducer – Product Description 103
Gamma Medica Inc Pipeline Products & Ongoing Clinical Trials Overview 104
Integrated Ultrasound Molecular Breast Imaging System – Product Status 104
Integrated Ultrasound Molecular Breast Imaging System – Product Description 104
GE HealthCare Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 105
Breast Assistant – Product Status 105
Breast Assistant – Product Description 105
Point-Of-Care Ultrasound System – Traumatic Injury – Product Status 106
Point-Of-Care Ultrasound System – Traumatic Injury – Product Description 106
Ultrasonic-Imaging Device – Product Status 106
Ultrasonic-Imaging Device – Product Description 107
Ultrasound Imaging Device – Portal Hypertension – Product Status 107
Ultrasound Imaging Device – Portal Hypertension – Product Description 107
GE Vingmed Ultrasound A.S. Pipeline Products & Ongoing Clinical Trials Overview 108
Ultrasound System – Heart Failure – Product Status 108
Ultrasound System – Heart Failure – Product Description 108
Harvard University Pipeline Products & Ongoing Clinical Trials Overview 109
Robot-Driven Ultrasound Imaging Catheter – Product Status 109
Robot-Driven Ultrasound Imaging Catheter – Product Description 109
H-Cubed Inc. Pipeline Products & Ongoing Clinical Trials Overview 110
Ultrasound Micro-Transducer – Product Status 110
Ultrasound Micro-Transducer – Product Description 110
HD Medical Group Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 111
Ultrasound Device – Product Status 111
Ultrasound Device – Product Description 111
Helix Medical Systems Ltd. Pipeline Products & Ongoing Clinical Trials Overview 112
CBUS 3000 – Product Status 112
CBUS 3000 – Product Description 112
IMGT Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 113
IMD10 – Product Status 113
IMD10 – Product Description 113
IMGT Co Ltd – Ongoing Clinical Trials Overview 114
IMD10 – A Prospective, Single Study to Evaluate the Efficacy and Safety of Folfirinox Prior Chemotherapy and Imd10 Combined Treatment in Patients with Locally Advanced or Borderline Resectable Pancreatic Cancer in Order to Evaluate the Drug Delivery Improvement Effect of the High-intensity Focused Ultrasound Surgical Device ‘IMD10’ Institutional, Controlled, Randomized, Single-blind (Independent Rater), Exploratory Trial 115
Indian Institute of Technology Madras Pipeline Products & Ongoing Clinical Trials Overview 116
Ultrasound-Based Temperature Tracking Solution – Product Status 116
Ultrasound-Based Temperature Tracking Solution – Product Description 116
Industrial Technology Research Institute Pipeline Products & Ongoing Clinical Trials Overview 117
Handheld Ultrasound System – Product Status 117
Handheld Ultrasound System – Product Description 117
InnerVision Medical Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 118
2D Transducer – Product Status 118
2D Transducer – Product Description 118
4D Imaging Ultrasound System – Product Status 119
4D Imaging Ultrasound System – Product Description 119
Flash Ultrasound – Product Status 119
Flash Ultrasound – Product Description 120
InnoMind Technology Corporation Pipeline Products & Ongoing Clinical Trials Overview 121
ComUltra – Product Status 121
ComUltra – Product Description 121
Insituvue, Inc Pipeline Products & Ongoing Clinical Trials Overview 122
SFH5000 – Product Status 122
SFH5000 – Product Description 122
SFP1000 – Product Status 123
SFP1000 – Product Description 123
Israel Institute of Technology Pipeline Products & Ongoing Clinical Trials Overview 124
Ultrasound Imaging System – Product Status 124
Ultrasound Imaging System – Product Description 124
Jiangsu Tingsheng Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 125
2D Intracardiac Ultrasound – Product Status 125
2D Intracardiac Ultrasound – Product Description 125
4D Intracardiac Ultrasound – Product Status 126
4D Intracardiac Ultrasound – Product Description 126
Johns Hopkins University Pipeline Products & Ongoing Clinical Trials Overview 127
Ultrasound-Based Anastomosis Detection And Assessment Tool – Product Status 127
Ultrasound-Based Anastomosis Detection And Assessment Tool – Product Description 127
Keter Medical systems Ltd Pipeline Products & Ongoing Clinical Trials Overview 128
Doppler TCD System – Product Status 128
Doppler TCD System – Product Description 128
King’s College Hospital NHS Foundation Trust Pipeline Products & Ongoing Clinical Trials Overview 129
The INSIGHT Scan – Product Status 129
The INSIGHT Scan – Product Description 129
King’s College Hospital NHS Foundation Trust – Ongoing Clinical Trials Overview 130
The INSIGHT Scan – Acceptability and Feasibility of Interprofessional Scheduled Whole-Body Point-of-Care Ultrasound in the Intensive Care Unit: A Randomised Controlled Feasibility Trial 131
Kitware Inc Pipeline Products & Ongoing Clinical Trials Overview 132
Multi-parametric Ultrasonic Elasticity Imaging System – Product Status 132
Multi-parametric Ultrasonic Elasticity Imaging System – Product Description 132
Koninklijke Philips NV Pipeline Products & Ongoing Clinical Trials Overview 133
AI Assisted Lumify Ultrasound – Product Status 133
AI Assisted Lumify Ultrasound – Product Description 133
Lenek Technologies Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 134
Portable Ultrasound Device – Product Status 134
Portable Ultrasound Device – Product Description 134
Lundquist Pipeline Products & Ongoing Clinical Trials Overview 135
Fetal Weight Assessing System – Product Status 135
Fetal Weight Assessing System – Product Description 135
Massachusetts Institute of Technology Pipeline Products & Ongoing Clinical Trials Overview 136
cUSBr-Patch – Product Status 136
cUSBr-Patch – Product Description 136
Non-Contact Laser Ultrasound System – Product Status 137
Non-Contact Laser Ultrasound System – Product Description 137
Portable Ultrasound Scanner – Product Status 137
Portable Ultrasound Scanner – Product Description 138
Mayo Clinic Pipeline Products & Ongoing Clinical Trials Overview 139
Multispectral Imaging and Elasticity Assessment Tool – Thyroid Nodule – Product Status 139
Multispectral Imaging and Elasticity Assessment Tool – Thyroid Nodule – Product Description 139
MediView XR Inc Pipeline Products & Ongoing Clinical Trials Overview 140
XR50 – Product Status 140
XR50 – Product Description 140
Menodys Inc. Pipeline Products & Ongoing Clinical Trials Overview 141
Ultrasound Imaging Device – Product Status 141
Ultrasound Imaging Device – Product Description 141
Nanyang Technological University Pipeline Products & Ongoing Clinical Trials Overview 142
3D Printed Ultrasound Device – Product Status 142
3D Printed Ultrasound Device – Product Description 142
National University of Science and Technology MiSiS Pipeline Products & Ongoing Clinical Trials Overview 143
Ultrasound Tomography System – Product Status 143
Ultrasound Tomography System – Product Description 143
Near Infrared Imaging, LLC Pipeline Products & Ongoing Clinical Trials Overview 144
Optical Ultrasound Tomography (PAT 2700) – Product Status 144
Optical Ultrasound Tomography (PAT 2700) – Product Description 144
Neos Newborn Solutions SL Pipeline Products & Ongoing Clinical Trials Overview 145
Neosonics – Meningitis – Product Status 145
Neosonics – Meningitis – Product Description 145
Neosonics – Peritonitis – Product Status 146
Neosonics – Peritonitis – Product Description 146
Neosonics – Uveitis – Product Status 146
Neosonics – Uveitis – Product Description 147
NerveVision, Inc. Pipeline Products & Ongoing Clinical Trials Overview 148
Brachial Plexus Ultrasound Needle Guidance System – Product Status 148
Brachial Plexus Ultrasound Needle Guidance System – Product Description 148
Neusoft Medical Systems Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 149
Phoenix System – Product Status 149
Phoenix System – Product Description 149
New York University Pipeline Products & Ongoing Clinical Trials Overview 150
Ultrasound Probe – Product Status 150
Ultrasound Probe – Product Description 150
North Carolina State University Pipeline Products & Ongoing Clinical Trials Overview 151
Ultrasonic Transducer Array – Product Status 151
Ultrasonic Transducer Array – Product Description 151
Vortex Ultrasound Transducer – Product Status 152
Vortex Ultrasound Transducer – Product Description 152
Norwegian University of Science and Technology Pipeline Products & Ongoing Clinical Trials Overview 153
Capacitive Micromachined Ultrasound Transducer – Product Status 153
Capacitive Micromachined Ultrasound Transducer – Product Description 153
NovaSignal Corp Pipeline Products & Ongoing Clinical Trials Overview 154
Transcranial Doppler Robotic System – Product Status 154
Transcranial Doppler Robotic System – Product Description 154
Novioscan Holdings BV Pipeline Products & Ongoing Clinical Trials Overview 155
SENS-U Adults Bladder Sensor – Product Status 155
SENS-U Adults Bladder Sensor – Product Description 155
OXEMS Limited Pipeline Products & Ongoing Clinical Trials Overview 156
Oxford Electromagnetic Acoustic Imaging – Product Status 156
Oxford Electromagnetic Acoustic Imaging – Product Description 156
OxSonics Ltd Pipeline Products & Ongoing Clinical Trials Overview 157
SonoTran System – Product Status 157
SonoTran System – Product Description 157
OxSonics Ltd – Ongoing Clinical Trials Overview 158
SonoTran System – First-in-human Study to Assess the Safety and Efficacy of SonoTran 159
Periomedic Ltd Pipeline Products & Ongoing Clinical Trials Overview 160
Periomedic Ultrasound Device – Product Status 160
Periomedic Ultrasound Device – Product Description 160
Periomedic Ltd – Ongoing Clinical Trials Overview 161
Periomedic Ultrasound Device – A Study to Validate Use of A Novel Ultrasound Device in Early Detection of Gingivitis 162
Philips Healthcare Pipeline Products & Ongoing Clinical Trials Overview 163
Ultrasound System – Product Status 163
Ultrasound System – Product Description 163
Whobus System – Product Status 163
Whobus System – Product Description 164
Philips Ultrasound, Inc. Pipeline Products & Ongoing Clinical Trials Overview 165
Affiniti CVx – Product Status 165
Affiniti CVx – Product Description 165
Physics for Medicine Paris Pipeline Products & Ongoing Clinical Trials Overview 166
Functional Ultrasound Imaging Tool – Product Status 166
Functional Ultrasound Imaging Tool – Product Description 166
Piur Imaging GmbH Pipeline Products & Ongoing Clinical Trials Overview 167
PIUR tUS Infinity System – Product Status 167
PIUR tUS Infinity System – Product Description 167
Pohang University of Science And Technology Pipeline Products & Ongoing Clinical Trials Overview 168
Ultrasound Device – Product Status 168
Ultrasound Device – Product Description 168
Provisio Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 169
SLT Endovascular Device – Product Status 169
SLT Endovascular Device – Product Description 169
PulseNmore Ltd Pipeline Products & Ongoing Clinical Trials Overview 170
Pulsenmore ES – Product Status 170
Pulsenmore ES – Product Description 171
Remote Follicular Monitoring Device – In-Vitro Fertilization (IVF) – Product Status 171
Remote Follicular Monitoring Device – In-Vitro Fertilization (IVF) – Product Description 171
Remote Monitoring Device – Chronic Heart Failure – Product Status 172
Remote Monitoring Device – Chronic Heart Failure – Product Description 172
Remote Monitoring Device – End-Stage Renal Disease (ESRD) – Product Status 172
Remote Monitoring Device – End-Stage Renal Disease (ESRD) – Product Description 173
PulseNmore Ltd – Ongoing Clinical Trials Overview 174
Pulsenmore ES – The Effect of Biweekly Follow up Along With at Home Ultrasound on the Anxiety of Pregnant Women Following Recurrent Pregnancy Loss 175
QB Sonic, Inc. Pipeline Products & Ongoing Clinical Trials Overview 176
Ultrasound Diagnostic System – Product Status 176
Ultrasound Diagnostic System – Product Description 176
QT Imaging Inc Pipeline Products & Ongoing Clinical Trials Overview 177
QT Ultrasound Breast Scanner-1 – Product Status 177
QT Ultrasound Breast Scanner-1 – Product Description 177
QT Ultrasound Breast Scanner-Young Women – Product Status 178
QT Ultrasound Breast Scanner-Young Women – Product Description 178
QT Ultrasound Infant Imaging Scanner – Product Status 178
QT Ultrasound Infant Imaging Scanner – Product Description 179
QT Ultrasound Orthopedic Imaging Scanner – Product Status 179
QT Ultrasound Orthopedic Imaging Scanner – Product Description 180
Rensselaer Polytechnic Institute Pipeline Products & Ongoing Clinical Trials Overview 181
Wearable Ultrasound Device – Product Status 181
Wearable Ultrasound Device – Product Description 181
Rimed Ltd Pipeline Products & Ongoing Clinical Trials Overview 182
Digi-Lite 2.0 – Product Status 182
Digi-Lite 2.0 – Product Description 182
Rivanna Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 183
Accuro – Joint Injection – Product Status 183
Accuro – Joint Injection – Product Description 183
Accuro – Nerve Blockage – Product Status 184
Accuro – Nerve Blockage – Product Description 184
Accuro – Thyroid Biopsy – Product Status 184
Accuro – Thyroid Biopsy – Product Description 185
Accuro – Vascular Access – Product Status 185
Accuro – Vascular Access – Product Description 185
Accuro 3S – Product Status 186
Accuro 3S – Product Description 186
Accuro XV – Product Status 186
Accuro XV – Product Description 187
Anesthesia Guidance System – Product Status 187
Anesthesia Guidance System – Product Description 187
Lumbar Puncture Guidance System – Product Status 188
Lumbar Puncture Guidance System – Product Description 188
Samsung Medison Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 189
HERA W10 Elite Diagnostic Software System – Hardware and Software Features – Product Status 189
HERA W10 Elite Diagnostic Software System – Hardware and Software Features – Product Description 190
Ultrasound And CAD Device – Product Status 190
Ultrasound And CAD Device – Product Description 190
Ultrasound Diagnostic Device – Embryonic Skeletal Muscles – Product Status 191
Ultrasound Diagnostic Device – Embryonic Skeletal Muscles – Product Description 191
Senosine Ltd Pipeline Products & Ongoing Clinical Trials Overview 192
Pulsar 1 – Product Status 192
Pulsar 1 – Product Description 192
Shanghai Shenzhi Information Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 193
AI Ultrasound App – Product Status 193
AI Ultrasound App – Product Description 193
Shanghai Yichao Medical Equipment Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 194
3D Ultrasound Diagnostic And Surgical Robot – Product Status 194
3D Ultrasound Diagnostic And Surgical Robot – Product Description 194
Shenzhen Mindray Bio-Medical Electronics Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 195
Artisona – Product Status 195
Artisona – Product Description 195
Consona – Product Status 196
Consona – Product Description 196
Emus – Product Status 196
Emus – Product Description 196
Hecho – Product Status 197
Hecho – Product Description 197
Velite – Product Status 197
Velite – Product Description 197
Shenzhen Xinhuan Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 198
4D ICE – Product Status 198
4D ICE – Product Description 198
Siemens Healthineers AG Pipeline Products & Ongoing Clinical Trials Overview 199
8C3 HD Ultrasound Probe – Product Status 199
8C3 HD Ultrasound Probe – Product Description 199
Siemens Medical Solutions USA Inc Pipeline Products & Ongoing Clinical Trials Overview 200
ACUSON Origin Diagnostic Ultrasound System – Product Status 200
ACUSON Origin Diagnostic Ultrasound System – Product Description 200
Singapore General Hospital Pipeline Products & Ongoing Clinical Trials Overview 201
Automated Bio-Imaging System – Product Status 201
Automated Bio-Imaging System – Product Description 201
Socionext Inc. Pipeline Products & Ongoing Clinical Trials Overview 202
viewphii-US – Product Status 202
viewphii-US – Product Description 202
SONARIUM MEDICAL Ltd. Pipeline Products & Ongoing Clinical Trials Overview 203
Breast Imaging System – Product Status 203
Breast Imaging System – Product Description 203
Sonavex Inc Pipeline Products & Ongoing Clinical Trials Overview 204
EchoSure System (ESV2) – Product Status 204
EchoSure System (ESV2) – Product Description 204
Sonavex Inc – Ongoing Clinical Trials Overview 205
EchoSure System (ESV2) – Maturation of Arteriovenous Fistula With Automated Sonography Assessments Trial 206
Sonivate Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 207
SonicEye – Abdominal Imaging – Product Status 207
SonicEye – Abdominal Imaging – Product Description 207
SonicEye – Circulatory System Assessment – Product Status 208
SonicEye – Circulatory System Assessment – Product Description 208
SonicEye – Fetal Imaging – Product Status 208
SonicEye – Fetal Imaging – Product Description 209
SonicEye – General Ultrasound Imaging – Product Status 209
SonicEye – General Ultrasound Imaging – Product Description 209
SonicEye – Intraoperative Imaging – Product Status 210
SonicEye – Intraoperative Imaging – Product Description 210
SonicEye – OB/GYN – Product Status 210
SonicEye – OB/GYN – Product Description 210
SonicEye – Primary Care – Product Status 211
SonicEye – Primary Care – Product Description 211
SonicEye – Trauma Assessment – Product Status 211
SonicEye – Trauma Assessment – Product Description 212
SonicEye – Urology – Product Status 212
SonicEye – Urology – Product Description 212
Sound Wave Innovation Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 213
LIPUS-Brain – Product Status 213
LIPUS-Brain – Product Description 213
Sound Wave Innovation Co Ltd – Ongoing Clinical Trials Overview 214
LIPUS-Brain – A Study to Evaluate the Efficacy and Safety of LIPUS-Brain Transcranial Low-intensity Pulsed-wave Ultrasound Device in Patients with Early Alzheimer’s Disease 215
Staffordshire University Pipeline Products & Ongoing Clinical Trials Overview 216
Ultrasound Based Diagnostic Tool – Diabetic Foot Ulcer – Product Status 216
Ultrasound Based Diagnostic Tool – Diabetic Foot Ulcer – Product Description 216
Stanford University Pipeline Products & Ongoing Clinical Trials Overview 217
Capsule Ultrasound Device – Product Status 217
Capsule Ultrasound Device – Product Description 217
Short-Lag Spatial Coherence (SLSC) Imaging – Product Status 218
Short-Lag Spatial Coherence (SLSC) Imaging – Product Description 218
TechniScan Inc Pipeline Products & Ongoing Clinical Trials Overview 219
Svara Warm Bath Ultrasound System – Product Status 219
Svara Warm Bath Ultrasound System – Product Description 219
TelehealthRobotics Pipeline Products & Ongoing Clinical Trials Overview 220
Tele-robotic Ultrasound System – Product Status 220
Tele-robotic Ultrasound System – Product Description 220
The University of British Columbia Pipeline Products & Ongoing Clinical Trials Overview 221
Ultrasound Guidance System – Product Status 221
Ultrasound Guidance System – Product Description 221
The University of Nottingham Pipeline Products & Ongoing Clinical Trials Overview 222
Phonon Probe – Product Status 222
Phonon Probe – Product Description 222
Thomas Jefferson University Pipeline Products & Ongoing Clinical Trials Overview 223
Contrast-Enhanced Ultrasound – Hepatocellular Carcinoma – Product Status 223
Contrast-Enhanced Ultrasound – Hepatocellular Carcinoma – Product Description 223
Transonic Imaging Inc Pipeline Products & Ongoing Clinical Trials Overview 224
MUST Device – Breast Cancer – Product Status 224
MUST Device – Breast Cancer – Product Description 224
UltraDerm Diagnostics Pipeline Products & Ongoing Clinical Trials Overview 225
UltraDerm Lite – Product Status 225
UltraDerm Lite – Product Description 225
UltraVision Inc Pipeline Products & Ongoing Clinical Trials Overview 226
Brain Trauma Monitoring Device – Product Status 226
Brain Trauma Monitoring Device – Product Description 226
University College London Pipeline Products & Ongoing Clinical Trials Overview 227
Optical Ultrasound System – Product Status 227
Optical Ultrasound System – Product Description 227
University Health Network Pipeline Products & Ongoing Clinical Trials Overview 228
Tri-Modal Imaging Device – Product Status 228
Tri-Modal Imaging Device – Product Description 228
University of California Berkeley Pipeline Products & Ongoing Clinical Trials Overview 229
Health-Care Ultrasonic Imaging System – Product Status 229
Health-Care Ultrasonic Imaging System – Product Description 229
Piezoelectric Micromachined Ultrasonic Transducer Device – Product Status 230
Piezoelectric Micromachined Ultrasonic Transducer Device – Product Description 230
University of California San Diego Pipeline Products & Ongoing Clinical Trials Overview 231
Photoacoustic Ultrasound Tool – Product Status 231
Photoacoustic Ultrasound Tool – Product Description 231
Ultrasono-Manometry Esophageal Function Assessment – Product Status 232
Ultrasono-Manometry Esophageal Function Assessment – Product Description 232
Ultrasound Probe – Product Status 232
Ultrasound Probe – Product Description 233
Wound Health Monitoring Device – Product Status 233
Wound Health Monitoring Device – Product Description 233
University of Connecticut Pipeline Products & Ongoing Clinical Trials Overview 234
Transvaginal Imaging Device – Product Status 234
Transvaginal Imaging Device – Product Description 234
University of Connecticut – Ongoing Clinical Trials Overview 235
Transvaginal Imaging Device – Transvaginal Ultrasound and Photoacoustic Imaging of the Ovary 236
University of Illinois at Urbana-Champaign Pipeline Products & Ongoing Clinical Trials Overview 237
Ultrasonic Biopsy Needle Shear Wave Imaging Device – Product Status 237
Ultrasonic Biopsy Needle Shear Wave Imaging Device – Product Description 237
University of Malaya Pipeline Products & Ongoing Clinical Trials Overview 238
Ultrasound Image Processing System – Osteoarthritis – Product Status 238
Ultrasound Image Processing System – Osteoarthritis – Product Description 238
University of Massachusetts Lowell Pipeline Products & Ongoing Clinical Trials Overview 239
Ultrasound Device – Product Status 239
Ultrasound Device – Product Description 239
University of Michigan Pediatric Device Consortium Pipeline Products & Ongoing Clinical Trials Overview 240
Pocket Sonic – Product Status 240
Pocket Sonic – Product Description 240
University of Minnesota Pipeline Products & Ongoing Clinical Trials Overview 241
Lung Biopsy Tool – Product Status 241
Lung Biopsy Tool – Product Description 241
University of North Carolina Pipeline Products & Ongoing Clinical Trials Overview 242
Ultrasound Device – Arterial Plaque – Product Status 242
Ultrasound Device – Arterial Plaque – Product Description 242
Glossary 414
![]()