Waldenstrom Macroglobulinemia (Lymphoplasmacytic Lymphoma) Clinical Trial Analysis by Phase, Trial Status, End Point, Sponsor Type and Region, 2023 Update
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Waldenstrom Macroglobulinemia Clinical Trial Report Overview
A total of 709 Waldenstrom Macroglobulinemia clinical trials were conducted as of December 2023. The Waldenstrom Macroglobulinemia clinical trial report provides a comprehensive understanding of the Waldenstrom Macroglobulinemia clinical trial scenario across regions, and countries (G7 & E7), along with insights into the various phases, trial status, and end points status. The report also includes information about the sponsor type and the prominent sponsors associated with the trials.
| Key Regions | · Asia-Pacific
· Europe · North America · Middle East and Africa · South and Central America |
| Key Countries | · The US
· China · Italy · France · The UK · Canada · Australia · Germany · Spain |
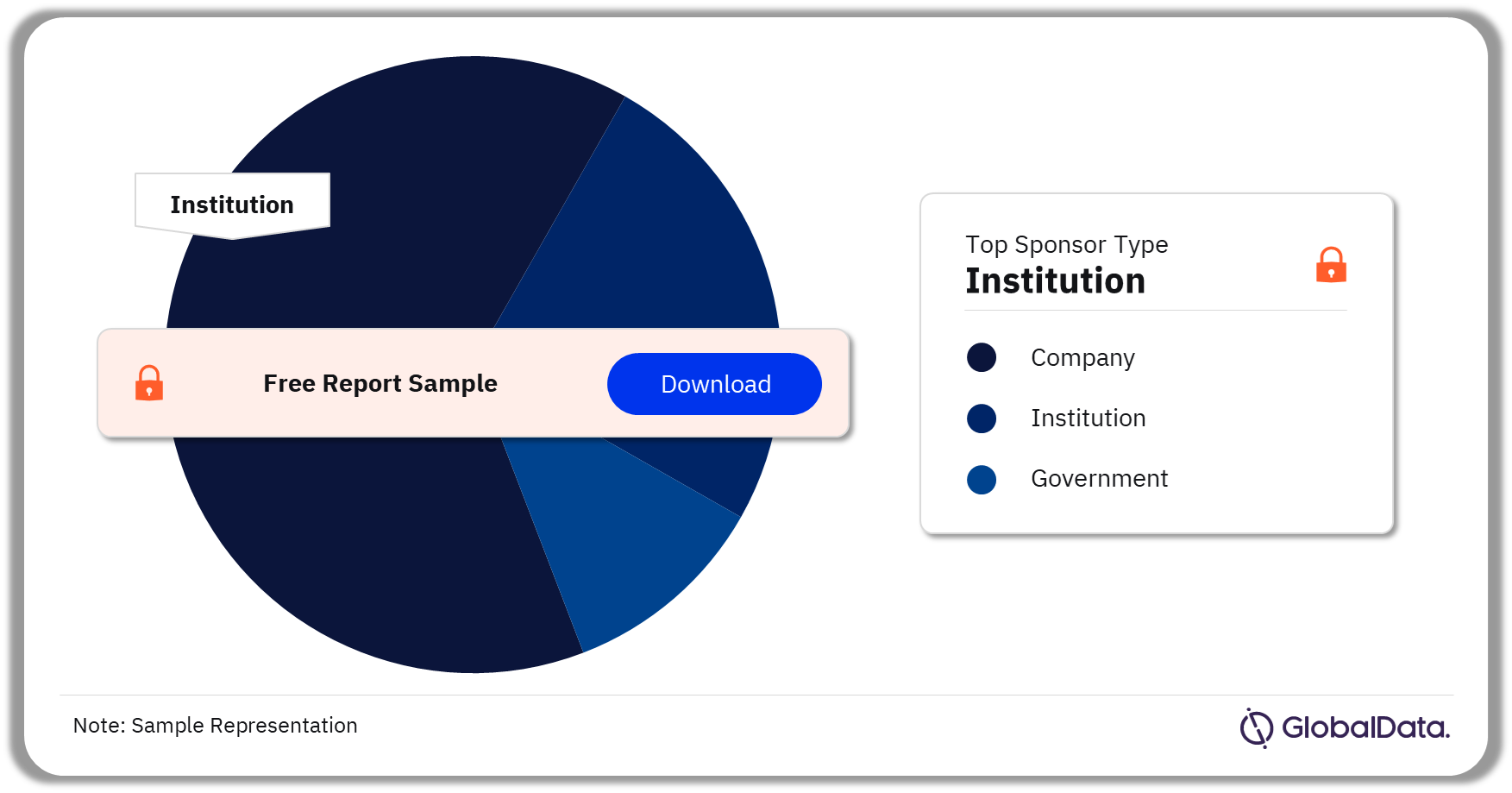
| Key Sponsor Types | · Company
· Institution · Government |
| Leading Sponsors | · Gilead Sciences Inc
· AbbVie Inc · Takeda Pharmaceutical Co Ltd · Johnson & Johnson · Bristol-Myers Squibb Co · F. Hoffmann-La Roche Ltd · BeiGene Ltd · AstraZeneca Plc · Others |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Waldenstrom Macroglobulinemia Clinical Trials Segmentation by Regions and Countries
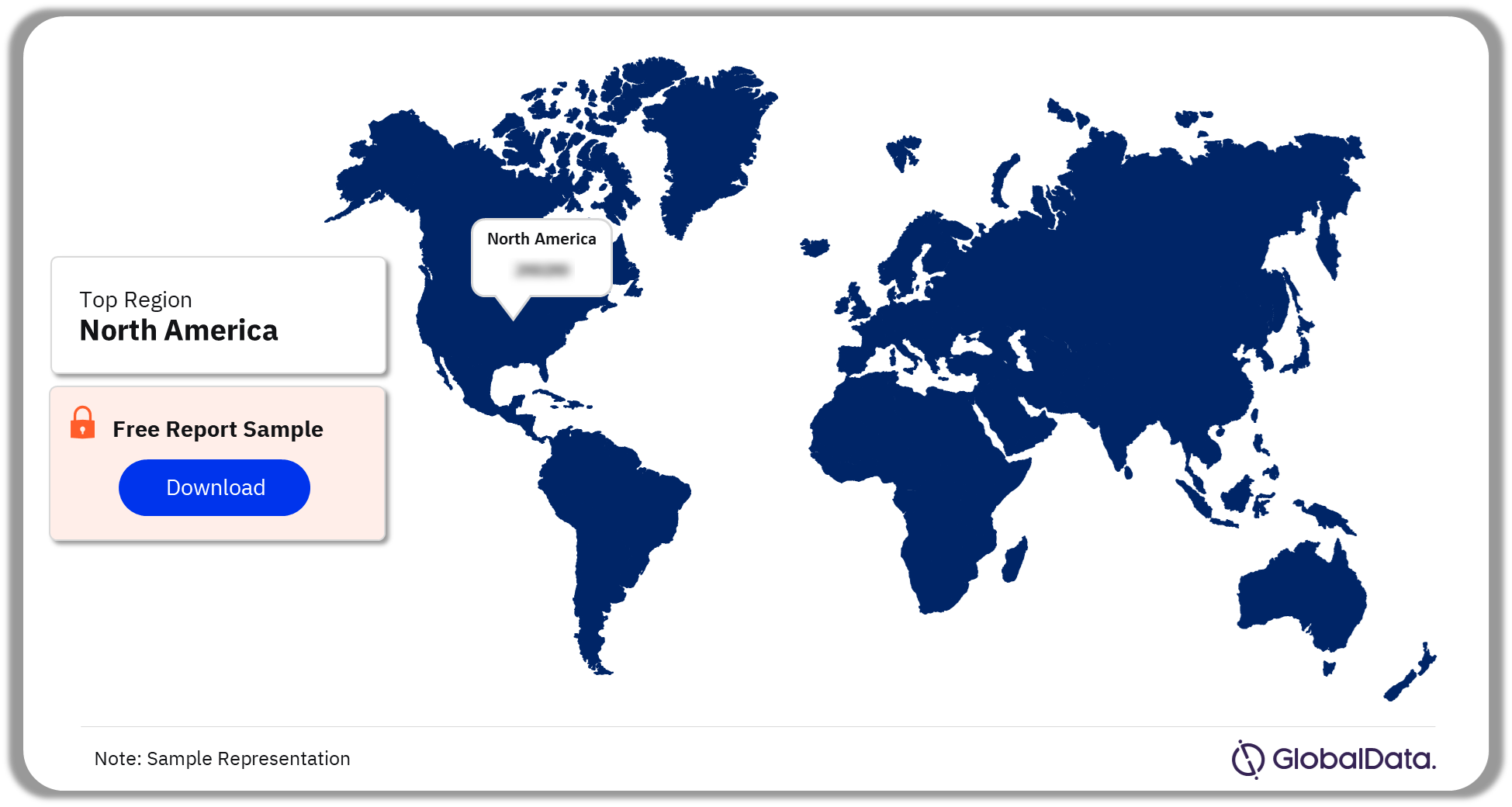
North America led the Waldenstrom Macroglobulinemia clinical trials landscape with more than 55% of the clinical trials conducted as of December 2023.
The key regions considered for Waldenstrom Macroglobulinemia clinical trials analysis are Asia-Pacific, Europe, North America, Middle East and Africa, and South and Central America.
In the country-wise analysis, the US had the highest number of Waldenstrom Macroglobulinemia (Lymphoplasmacytic Lymphoma) clinical trials, as of December 2023. Germany had the highest average patient enrollment of Waldenstrom Macroglobulinemia (Lymphoplasmacytic Lymphoma) clinical trials, as of December 2023.
G7 countries: Among the G7 (United States, United Kingdom, Germany, France, Italy, Canada and Japan) countries, the US had the highest proportion of Waldenstrom Macroglobulinemia (Lymphoplasmacytic Lymphoma) to oncology clinical trials as of December 2023.
E7 countries: Among the E7 (Brazil, Russia, India, China, Mexico, Turkey and Indonesia) countries, Turkey had the highest proportion of Waldenstrom Macroglobulinemia (Lymphoplasmacytic Lymphoma) to Oncology clinical trials as of December 2023.
Waldenstrom Macroglobulinemia Clinical Trials Analysis by Regions, 2023 (%)
Buy the Full Report for More Regional Insights into the Waldenstrom Macroglobulinemia Clinical Trials
Waldenstrom Macroglobulinemia Clinical Trials Segmentation by Sponsor Types
The key sponsor types for Waldenstrom Macroglobulinemia clinical trials are company, institution, and the government. As of December 2023, more than 50% of Waldenstrom Macroglobulinemia clinical trials conducted were sponsored by institution sponsor type, followed by the company.
Waldenstrom Macroglobulinemia Clinical Trials Analysis by Sponsor Type, 2023 (%)
Buy the Full Report for More Insights into the Waldenstrom Macroglobulinemia Clinical Trials Sponsor Types
Waldenstrom Macroglobulinemia Clinical Trials - Competitive Landscape
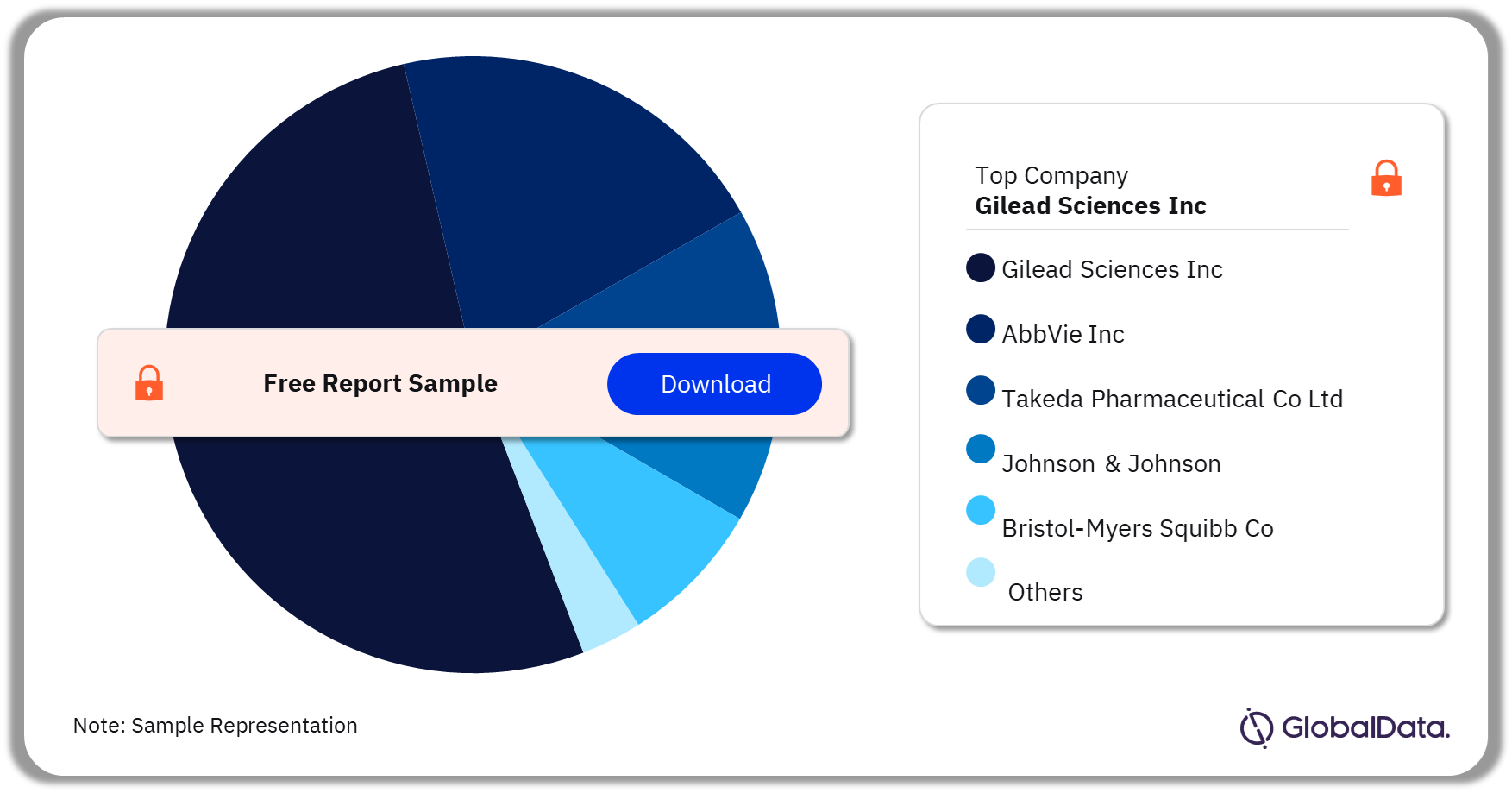
In 2023, Gilead Sciences Inc. conducted the highest number of Waldenstrom Macroglobulinemia clinical trials.
A few of the leading sponsors for Waldenstrom Macroglobulinemia clinical trials are:
- Gilead Sciences Inc
- AbbVie Inc
- Takeda Pharmaceutical Co Ltd
- Johnson & Johnson
- Bristol-Myers Squibb Co
- Hoffmann-La Roche Ltd
- BeiGene Ltd
- AstraZeneca Plc
Waldenstrom Macroglobulinemia Clinical Trials Analysis by Leading Companies, 2023 (%)
Buy Full Report to Know More About the Leading Players of Waldenstrom Macroglobulinemia Clinical Trials
Segments Covered in the Report
Waldenstrom Macroglobulinemia Clinical Trials Regional Outlook (2023)
- Asia-Pacific
- Europe
- North America
- Middle East and Africa
- South and Central America
Waldenstrom Macroglobulinemia Clinical Trials Country Outlook (2023)
- The US
- China
- Italy
- France
- The UK
- Canada
- Australia
- Germany
- Spain
Waldenstrom Macroglobulinemia Clinical Trials Sponsor Type Outlook (2023)
- Company
- Institution
- Government
Scope
- The report provides a snapshot of the global clinical trials landscape.
- The report provides top-level data related to the clinical trials by region, country (G7 & E7), trial status, trial phase, sponsor type, and endpoint status.
- The report reviews the top companies involved and enlists all trials (trial title, phase, and status) of the company.
- The report provides all the unaccomplished trials (terminated, suspended, and withdrawn) with a reason for accomplishment.
- The report provides enrollment trends for the past five years.
- The report provides the latest news for the past three months.
Reasons to Buy
- Assists in formulating key investment strategies.
- Helps in identifying time-saving and cost-efficient locations to conduct clinical trials.
- Provides top-level analysis of the global clinical trials market to help in identifying key business opportunities.
- Helps in understanding trial count and enrollment trends by country in the global therapeutics market.
- Aids in interpreting the success rates of clinical trials by providing a comparative scenario of completed and uncompleted (terminated, suspended, or withdrawn) trials.
- Facilitates clinical trial assessment of the indication on a global, regional, and country level.
AbbVie Inc
Takeda Pharmaceutical Co Ltd
Johnson & Johnson
Bristol-Myers Squibb Co
F. Hoffmann-La Roche Ltd
BeiGene Ltd
AstraZeneca Plc
Amgen Inc
Merck & Co Inc
Table of Contents
Table
Figures
Frequently asked questions
-
Which region accounted for the highest number of Waldenstrom Macroglobulinemia clinical trials in December 2023?
North America accounted for the highest number of Waldenstrom Macroglobulinemia clinical trials as of December 2023.
-
Which country conducted the highest number of Waldenstrom Macroglobulinemia clinical trials in December 2023?
The US conducted the highest number of Waldenstrom Macroglobulinemia clinical trials as of December 2023.
-
Which sponsor type was the most prominent in the Waldenstrom Macroglobulinemia clinical trials in December 2023?
As of December 2023, most of the Waldenstrom Macroglobulinemia clinical trials conducted were sponsored by institution sponsor type.
-
Who are the leading sponsors of Waldenstrom Macroglobulinemia clinical trials?
A few of the leading sponsors of Waldenstrom Macroglobulinemia clinical trials are Gilead Sciences Inc, AbbVie Inc, Takeda Pharmaceutical Co Ltd, Johnson & Johnson, Bristol-Myers Squibb Co, F. Hoffmann-La Roche Ltd, BeiGene Ltd, and AstraZeneca Plc among others.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.