Aesthetic Injectables – Pipeline Products by Stage of Development 17
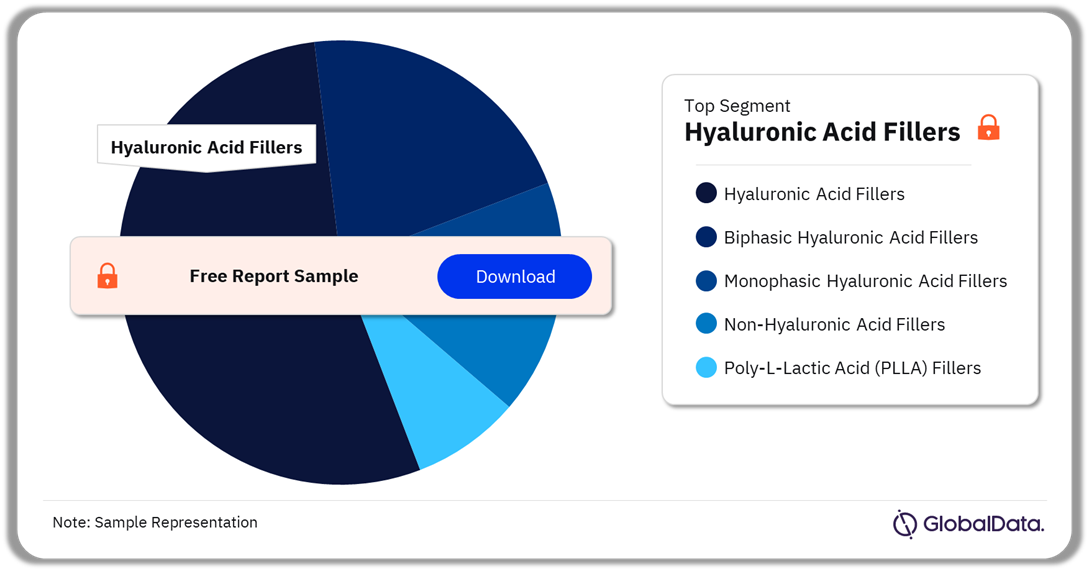
Aesthetic Injectables – Pipeline Products by Segment 18
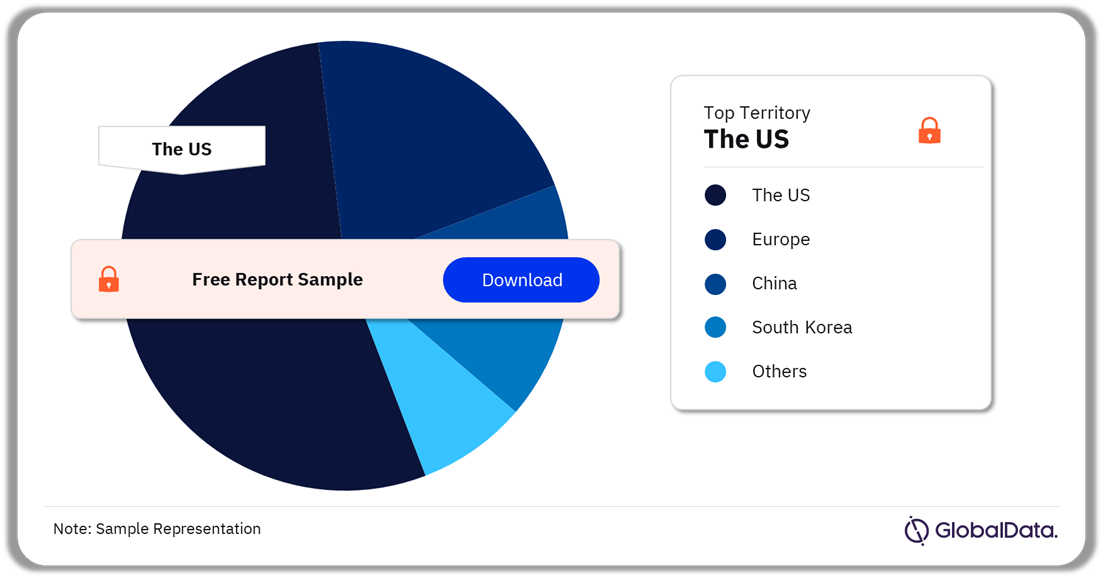
Aesthetic Injectables – Pipeline Products by Territory 19
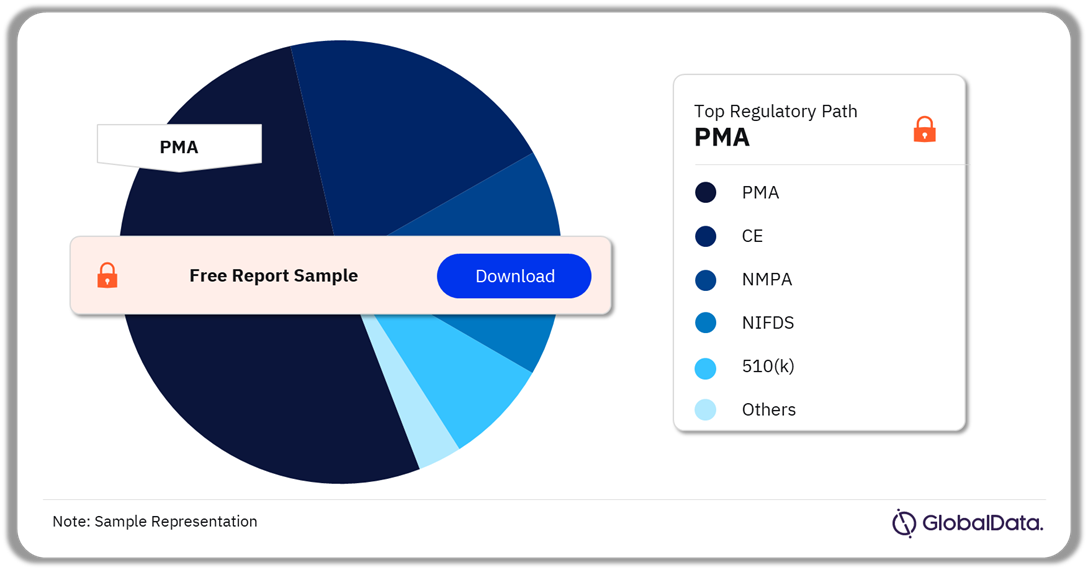
Aesthetic Injectables – Pipeline Products by Regulatory Path 21
Aesthetic Injectables – Pipeline Products by Estimated Approval Date 22
Aesthetic Injectables – Ongoing Clinical Trials 23
Aesthetic Injectables Companies – Pipeline Products by Stage of Development 24
Aesthetic Injectables – Pipeline Products by Stage of Development 27
Acro Biomedical Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 31
ACROFILL – Product Status 31
ACROFILL – Product Description 31
Advanced Aesthetic Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 32
Algeness DF – Product Status 32
Algeness DF – Product Description 32
Algeness HD – Product Status 33
Algeness HD – Product Description 33
Algeness LD – Product Status 33
Algeness LD – Product Description 34
Algeness VL – Product Status 34
Algeness VL – Product Description 34
Advanced Aesthetic Technologies Inc – Ongoing Clinical Trials Overview 35
Algeness VL – A Pivotal Clinical Study to Evaluate the Effectiveness and Safety of Algeness VL for the Correction of Moderate to Severe Nasolabial Folds 36
Algeness VL – A Prospective, Multi-center, Randomized, Controlled, Single-blind Study of the Safety and Effectiveness of Algeness DF 3.5% Deep Volumizing Filler to Correct Age-related Volume Deficit in the Mid-face 36
Aeon Astron Corporation Pipeline Products & Ongoing Clinical Trials Overview 37
BioDermal Filler – Product Status 37
BioDermal Filler – Product Description 37
Aesthetic Medical Device Pipeline Products & Ongoing Clinical Trials Overview 38
Alluris – Product Status 38
Alluris – Product Description 38
Skinlumina – Product Status 39
Skinlumina – Product Description 39
SkinTechMD – Product Status 39
SkinTechMD – Product Description 40
Allergan Aesthetics Pipeline Products & Ongoing Clinical Trials Overview 41
AAM SubQ Body Filler – Product Status 41
AAM SubQ Body Filler – Product Description 42
ADM Subdermal Body Filler – Product Status 42
ADM Subdermal Body Filler – Product Description 42
Elastagen – Acne Scars – Product Status 42
Elastagen – Acne Scars – Product Description 43
Elastagen – Skin Quality – Product Status 43
Elastagen – Skin Quality – Product Description 43
HA Threads – Product Status 43
HA Threads – Product Description 44
Juvederm – Decolletage – Product Status 44
Juvederm – Decolletage – Product Description 44
Juvederm – Hands – Product Status 44
Juvederm – Hands – Product Description 45
Juvederm – Neck Lines – Product Status 45
Juvederm – Neck Lines – Product Description 45
Juvederm RejuveCross – Deep Wrinkles – Product Status 45
Juvederm RejuveCross – Deep Wrinkles – Product Description 46
Juvederm RejuveCross – Etched Lines Wrinkles – Product Status 46
Juvederm RejuveCross – Etched Lines Wrinkles – Product Description 46
Juvederm RejuveCross Volumize – Cheek Augmentation – Product Status 46
Juvederm RejuveCross Volumize – Cheek Augmentation – Product Description 47
Juvederm Voluma – Nose – Product Status 47
Juvederm Voluma – Nose – Product Description 47
Juvederm Voluma Global – Malar Augmentation – Product Status 47
Juvederm Voluma Global – Malar Augmentation – Product Description 48
JUVEDERM VOLUMA XC – Temple Augmentation – Product Status 48
JUVEDERM VOLUMA XC – Temple Augmentation – Product Description 48
Allergan Aesthetics – Ongoing Clinical Trials Overview 49
Juvederm Voluma Global – Malar Augmentation – Clinical, Instrumental and Histological Evaluation of the Combined Use of Onabotulinumtoxin A and Hyaluronic Acid Fillers in Patients with Facial Paralysis 50
Almirall Ltd Pipeline Products & Ongoing Clinical Trials Overview 51
Hyaluronic Acid Facial Filler – Product Status 51
Hyaluronic Acid Facial Filler – Product Description 51
Aquavit Pharmaceuticals Inc Pipeline Products & Ongoing Clinical Trials Overview 52
Aquabelo – Product Status 52
Aquabelo – Product Description 52
Beijing MeiYan KongJian Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 53
Complex Collagen Filler (Type III) – Product Status 53
Complex Collagen Filler (Type III) – Product Description 53
First Generation PLLA Filler (Type III) – Product Status 54
First Generation PLLA Filler (Type III) – Product Description 54
Second Generation PCL Filler (Type III) – Product Status 54
Second Generation PCL Filler (Type III) – Product Description 55
BioPlus Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 56
HyalDew All – Product Status 56
HyalDew All – Product Description 56
HyalDew Fine – Product Status 57
HyalDew Fine – Product Description 57
HyalDew Shine – Product Status 57
HyalDew Shine – Product Description 58
HyalDew-Mid – Product Status 58
HyalDew-Mid – Product Description 58
BioSmart Nanotechnology Ltda Pipeline Products & Ongoing Clinical Trials Overview 59
Hyaluronic Acid Dermal Filler – Product Status 59
Hyaluronic Acid Dermal Filler – Product Description 59
Bioxis Pharmaceuticals Pipeline Products & Ongoing Clinical Trials Overview 60
Cytosmile – Product Status 60
Cytosmile – Product Description 60
MTI-12 – Product Status 61
MTI-12 – Product Description 61
MTI-12 – Surgical Repair – Product Status 61
MTI-12 – Surgical Repair – Product Description 62
BMG Pharma SpA Pipeline Products & Ongoing Clinical Trials Overview 63
SGA300 – Product Status 63
SGA300 – Product Description 63
SGA302 – Product Status 64
SGA302 – Product Description 64
Bmi Korea Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 65
BMI4002 – Product Status 65
BMI4002 – Product Description 65
CellPraxis Pipeline Products & Ongoing Clinical Trials Overview 66
Lipage – Product Status 66
Lipage – Product Description 66
Celltrix AB Pipeline Products & Ongoing Clinical Trials Overview 67
Credurance – Product Status 67
Credurance – Product Description 67
Collplant Biotechnologies Ltd Pipeline Products & Ongoing Clinical Trials Overview 68
rhCollagen Based Photocurable Regenerative Dermal Filler – Product Status 68
rhCollagen Based Photocurable Regenerative Dermal Filler – Product Description 68
rhCollagen Based Soft Tissue Filler – Product Status 69
rhCollagen Based Soft Tissue Filler – Product Description 69
Croma-Pharma GmbH Pipeline Products & Ongoing Clinical Trials Overview 70
Saypha Volume Plus Lidocaine – Product Status 70
Saypha Volume Plus Lidocaine – Product Description 70
Croma-Pharma GmbH – Ongoing Clinical Trials Overview 71
Saypha Volume Plus Lidocaine – A Randomized, Multi-center, Evaluator-blinded, Parallel Assignment, Active Controlled Study to Evaluate the Efficacy and Safety of Princess Voluma plus and Restylane Perlane Lidocaine for Correction of Midface Volume Deficit And/Or Midface Contour Deficiency 72
Dexlevo Inc Pipeline Products & Ongoing Clinical Trials Overview 73
Gouri – Product Status 73
Gouri – Product Description 73
Dongkook Pharmaceutical Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 74
DKB-119 – Product Status 74
DKB-119 – Product Description 74
DKB-133 – Product Status 75
DKB-133 – Product Description 75
DKM-410 – Product Status 75
DKM-410 – Product Description 76
DKM-423 – Product Status 76
DKM-423 – Product Description 76
EmulTech BV Pipeline Products & Ongoing Clinical Trials Overview 77
SC Injectable Collagen Stimulator – Product Status 77
SC Injectable Collagen Stimulator – Product Description 77
Establishment Labs SA Pipeline Products & Ongoing Clinical Trials Overview 78
Motiva Injector – Product Status 78
Motiva Injector – Product Description 78
Evolus Inc Pipeline Products & Ongoing Clinical Trials Overview 79
Evolysse Eye Infraorbital Hollows Filler – Product Status 79
Evolysse Eye Infraorbital Hollows Filler – Product Description 80
Evolysse Lift Nasolabial Folds Filler – Product Status 80
Evolysse Lift Nasolabial Folds Filler – Product Description 80
Evolysse Lips Volumizing And Countering Filler – Product Status 81
Evolysse Lips Volumizing And Countering Filler – Product Description 81
Evolysse Sculpt Mid Face Volume Filler – Product Status 81
Evolysse Sculpt Mid Face Volume Filler – Product Description 82
Evolysse Smooth Nasolabial Folds Filler – Product Status 82
Evolysse Smooth Nasolabial Folds Filler – Product Description 82
Fillmed Laboratoires Pipeline Products & Ongoing Clinical Trials Overview 83
NCTF 135 – Product Status 83
NCTF 135 – Product Description 83
NCTF 135 HA – Product Status 84
NCTF 135 HA – Product Description 84
G2GBIO Inc Pipeline Products & Ongoing Clinical Trials Overview 85
GB-3001 – Product Status 85
GB-3001 – Product Description 85
Galderma SA Pipeline Products & Ongoing Clinical Trials Overview 86
Biostimulator Filler – Product Status 86
Biostimulator Filler – Product Description 86
Hallura Ltd Pipeline Products & Ongoing Clinical Trials Overview 87
BiOLinkMatrix Gel – Product Status 87
BiOLinkMatrix Gel – Product Description 87
Hyaluronic Acid Dermal Filler – Product Status 88
Hyaluronic Acid Dermal Filler – Product Description 88
Histogen Inc Pipeline Products & Ongoing Clinical Trials Overview 89
HST002 – Product Status 89
HST002 – Product Description 89
Hugel Inc Pipeline Products & Ongoing Clinical Trials Overview 90
The Chaeum Pure No.1 – Product Status 90
The Chaeum Pure No.1 – Product Description 91
The Chaeum Pure No.2 – Product Status 91
The Chaeum Pure No.2 – Product Description 91
The Chaeum Pure No.3 – Product Status 92
The Chaeum Pure No.3 – Product Description 92
The Chaeum Pure No.4 – Product Status 92
The Chaeum Pure No.4 – Product Description 93
The Chaeum Style – Product Status 93
The Chaeum Style – Product Description 93
Innova Medical Design Pipeline Products & Ongoing Clinical Trials Overview 94
Aesthetic Injection – Product Status 94
Aesthetic Injection – Product Description 94
Innovia LLC Pipeline Products & Ongoing Clinical Trials Overview 95
Wrinkle Filler – Product Status 95
Wrinkle Filler – Product Description 95
inSoma Bio Inc Pipeline Products & Ongoing Clinical Trials Overview 96
Fractomer – Product Status 96
Fractomer – Product Description 96
Inventage Lab Inc Pipeline Products & Ongoing Clinical Trials Overview 97
IVL-MD-B001 – Product Status 97
IVL-MD-B001 – Product Description 97
IVL-MD-F001 – Product Status 98
IVL-MD-F001 – Product Description 98
IVL-MD-F002 – Product Status 98
IVL-MD-F002 – Product Description 99
Jinwoo Bio Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 100
HA Filler – Product Status 100
HA Filler – Product Description 100
HA Thread Filler – Product Status 101
HA Thread Filler – Product Description 101
Wool Filler – Product Status 102
Wool Filler – Product Description 102
Juvenis Ltd Pipeline Products & Ongoing Clinical Trials Overview 103
Tenergel – Product Status 103
Tenergel – Product Description 103
Khorionyx SA Pipeline Products & Ongoing Clinical Trials Overview 104
Autologous Globin Dermal Filler – Product Status 104
Autologous Globin Dermal Filler – Product Description 104
KiOmed Pharma Pipeline Products & Ongoing Clinical Trials Overview 105
KIO015 Intradermal Injectable Device – Product Status 105
KIO015 Intradermal Injectable Device – Product Description 105
KiOmed Pharma – Ongoing Clinical Trials Overview 106
KIO015 Intradermal Injectable Device – A Randomized, Controlled, Split-Face Study to Evaluate the Clinical Performance and Safety of KIO015 for Improving Face Skin Hydration 107
Laboratoires Vivacy Pipeline Products & Ongoing Clinical Trials Overview 108
IPN-21-SENSE Dermal Filler – Product Status 108
IPN-21-SENSE Dermal Filler – Product Description 108
Laboratoires Vivacy – Ongoing Clinical Trials Overview 109
IPN-21-SENSE Dermal Filler – A Multicenter, Prospective, Randomized Controlled Clinical Study of IPN-21-SENSE, a Novel Hyaluronic Acid Based Gel for Volume Deficiency in the Mid-face 110
LG Chem Ltd Pipeline Products & Ongoing Clinical Trials Overview 111
Dermal Filler (LR19059) – Product Status 111
Dermal Filler (LR19059) – Product Description 112
Dermal Filler (LR19093) – Product Status 112
Dermal Filler (LR19093) – Product Description 112
Hyaluronic Acid Filler (LR19094) – Product Status 112
Hyaluronic Acid Filler (LR19094) – Product Description 113
Hyaluronic Acid Filler (LR20023) – Product Status 113
Hyaluronic Acid Filler (LR20023) – Product Description 113
Hyaluronic Acid Filler (LR20024) – Product Status 114
Hyaluronic Acid Filler (LR20024) – Product Description 114
Next Generation Hyaluronic Acid Filler (LR20008) – Product Status 114
Next Generation Hyaluronic Acid Filler (LR20008) – Product Description 115
YVOIRE Y-Solution 360 – Product Status 115
YVOIRE Y-Solution 360 – Product Description 115
YVOIRE Y-Solution 540 – Product Status 116
YVOIRE Y-Solution 540 – Product Description 116
YVOIRE Y-Solution 720 – Product Status 116
YVOIRE Y-Solution 720 – Product Description 117
LG Chem Ltd – Ongoing Clinical Trials Overview 118
YVOIRE Y-Solution 360 – A Multicenter, Randomized, Rater-Blinded, No-Treatment Control Design Clinical Investigation to Evaluate the Effectiveness and Safety of YVOIRE Y-Solution 360 for Lip Augmentation 119
YVOIRE Y-Solution 540 – A Randomized, Multicenter, Evaluator-blinded, Active-controlled, Parallel-group Design Investigation to Evaluate the Effectiveness and Safety of YVOIRE Y-Solution 540 Versus YVOIRE Volume Plus in Nasolabial Folds Injection 120
LifeSprout Inc Pipeline Products & Ongoing Clinical Trials Overview 121
Lumina – Product Status 121
Lumina – Product Description 121
LifeSprout Inc – Ongoing Clinical Trials Overview 122
Lumina – A Prospective, Randomized, Controlled, Multi-center Study of the Safety and Effectiveness of Lumina in the Treatment of Nasolabial Folds 123
Medytox Inc Pipeline Products & Ongoing Clinical Trials Overview 124
MT943 – Product Status 124
MT943 – Product Description 124
Neuramis Deep Lidocaine – Product Status 125
Neuramis Deep Lidocaine – Product Description 125
Neuramis Volume Lidocaine – Product Status 125
Neuramis Volume Lidocaine – Product Description 126
Merz Aesthetics Pipeline Products & Ongoing Clinical Trials Overview 127
VP1 Lido US – Product Status 127
VP1 Lido US – Product Description 127
Mesoestetic Pharma Group SL Pipeline Products & Ongoing Clinical Trials Overview 128
LIFT001 – Product Status 128
LIFT001 – Product Description 128
mRDX-02-17 – Product Status 129
mRDX-02-17 – Product Description 129
Moma Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 130
Restogel – 131 – Product Status 130
Restogel – 131 – Product Description 130
N8 Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 131
Ceragenin-Infused Dermal Filler – Product Status 131
Ceragenin-Infused Dermal Filler – Product Description 131
Oligo Medic Inc Pipeline Products & Ongoing Clinical Trials Overview 132
AntaRep – Product Status 132
AntaRep – Product Description 132
Oxartis Ltd Pipeline Products & Ongoing Clinical Trials Overview 133
Intra-Dermal Filler – Product Status 133
Intra-Dermal Filler – Product Description 133
PB&B SA Pipeline Products & Ongoing Clinical Trials Overview 134
PB&B Facial Volumizer – Product Status 134
PB&B Facial Volumizer – Product Description 134
Peptron Inc Pipeline Products & Ongoing Clinical Trials Overview 135
PLA Dermal Filler – Product Status 135
PLA Dermal Filler – Product Description 135
PetVivo Holdings Inc Pipeline Products & Ongoing Clinical Trials Overview 136
CosmetaLife – Product Status 136
CosmetaLife – Product Description 136
Dermal Filler – Lip – Product Status 137
Dermal Filler – Lip – Product Description 137
Q-Med AB Pipeline Products & Ongoing Clinical Trials Overview 138
GAL1906 – Product Status 138
GAL1906 – Product Description 138
GP0112 – Product Status 139
GP0112 – Product Description 139
Samyang Biopharmaceuticals Corp Pipeline Products & Ongoing Clinical Trials Overview 140
Aesthetic Filler – Product Status 140
Aesthetic Filler – Product Description 140
Lafullen Dermal Filler – Product Status 141
Lafullen Dermal Filler – Product Description 141
SYB Filler (SF-01) – Product Status 141
SYB Filler (SF-01) – Product Description 142
Samyang Biopharmaceuticals Corp – Ongoing Clinical Trials Overview 143
Lafullen Dermal Filler – A Randomized, Evaluator-blind, Matched Pairs, Prospective, Non Inferiority, Confirmatory Study to Compare the Safety and Efficacy Between Lafullen15 and Lafullen in the Correction of Nasolabial Folds 144
Samyang Holdings Corp Pipeline Products & Ongoing Clinical Trials Overview 145
SYM-1912 – Product Status 145
SYM-1912 – Product Description 145
SYM-2005 – Product Status 146
SYM-2005 – Product Description 146
SYM-2303 – Product Status 146
SYM-2303 – Product Description 146
Sebana Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 147
Dermal Filler – Product Status 147
Dermal Filler – Product Description 147
Shanghai Haohai Biological Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 148
Organic Cross-Linked HA Dermal Filler – Product Status 148
Organic Cross-Linked HA Dermal Filler – Product Description 148
Shin Poong Pharm Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 149
FreeWink – Product Status 149
FreeWink – Product Description 149
Silk Medical Aesthetics Inc Pipeline Products & Ongoing Clinical Trials Overview 150
Dermal Filler – Product Status 150
Dermal Filler – Product Description 150
SMA-001 – Product Status 151
SMA-001 – Product Description 151
Sinclair Pharma Ltd Pipeline Products & Ongoing Clinical Trials Overview 152
Atlean – Product Status 152
Atlean – Product Description 152
Ellanse Lidocaine – Product Status 153
Ellanse Lidocaine – Product Description 153
MaiLi – Product Status 153
MaiLi – Product Description 154
MaiLi Extreme – Product Status 154
MaiLi Extreme – Product Description 154
Perfectha G – Product Status 155
Perfectha G – Product Description 155
Sinclair Pharma Ltd – Ongoing Clinical Trials Overview 156
Ellanse Lidocaine – Prospective, Single Site, Controlled Comparative Study to Identify and Assess Tissue Responses After Injection With Various Biostimulatory Products 157
MaiLi Extreme – A Post Market Clinical Follow Up (PMCF) Study to Assess the Safety and Efficacy of MaiLi Volume and MaiLi Extreme in the Treatment of Temporal Hollowing, Mid-Face Volume Deficit, Jawline Ptosis, and Chin Retrusion 158
Sofregen Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 159
Silk Protein – Tissue/Dermal Filler – Product Status 159
Silk Protein – Tissue/Dermal Filler – Product Description 159
So-Young International Inc Pipeline Products & Ongoing Clinical Trials Overview 160
Extracellular Matrix Bio Gel Injectable Filler – Product Status 160
Extracellular Matrix Bio Gel Injectable Filler – Product Description 160
Spiderwort Pipeline Products & Ongoing Clinical Trials Overview 161
CelluJuve – Product Status 161
CelluJuve – Product Description 161
SunMax Biotechnology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 162
Sunmax FULLSGEN With Lidocaine – Product Status 162
Sunmax FULLSGEN With Lidocaine – Product Description 162
SwiftPharma BV Pipeline Products & Ongoing Clinical Trials Overview 163
Injectable Dermal Filler – Product Status 163
Injectable Dermal Filler – Product Description 163
TEI Biosciences Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 164
Injectable Dermal Filler – Product Status 164
Injectable Dermal Filler – Product Description 164
Tempo Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 165
MAP Tissue Scaffold-Based Dermal Filler – Product Status 165
MAP Tissue Scaffold-Based Dermal Filler – Product Description 165
Teoxane SA Pipeline Products & Ongoing Clinical Trials Overview 166
Next Generation Dermal Filler – Product Status 166
Next Generation Dermal Filler – Product Description 166
Teosyal RHA 1 – Product Status 167
Teosyal RHA 1 – Product Description 167
Teoxane SA – Ongoing Clinical Trials Overview 168
Teosyal RHA 1 – A Prospective, Single Blind, Single-center Study Evaluating the Histology and Intradermal Implantation of the Teosyal RHA Collection of Fillers 169
Tetratherix Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 170
Tetrafill – Product Status 170
Tetrafill – Product Description 170
Tissueform Inc Pipeline Products & Ongoing Clinical Trials Overview 171
NatruDerme – Product Status 171
NatruDerme – Product Description 171
PureVoluma – Product Status 172
PureVoluma – Product Description 172
TruElastin Laboratories Inc Pipeline Products & Ongoing Clinical Trials Overview 173
TEL219 – Product Status 173
TEL219 – Product Description 173
Trylle Skin Health Inc Pipeline Products & Ongoing Clinical Trials Overview 174
Collagen-Based Dermal Filler – Product Status 174
Collagen-Based Dermal Filler – Product Description 174
University of California Santa Barbara Pipeline Products & Ongoing Clinical Trials Overview 175
Needle Free Injector – Product Status 175
Needle Free Injector – Product Description 175
University of Missouri Pipeline Products & Ongoing Clinical Trials Overview 176
Collagen-Based Dermal Filler – Product Status 176
Collagen-Based Dermal Filler – Product Description 176
Volumina Medical SA Pipeline Products & Ongoing Clinical Trials Overview 177
Adipearl – Product Status 177
Adipearl – Product Description 177
Volumina Medical SA – Ongoing Clinical Trials Overview 178
Adipearl – A Single-Arm Pilot Study to Evaluate the Safety of Adipearl Injection in the Subcutaneous Tissues of Adults 179
Adipearl – A Single-Arm, Prospective, Clinical Investigation Evaluating the Safety and Feasibility of Adipearl Implantation in the Face of Adult Subjects 179
Glossary 205
![]()