Bone Growth Stimulators Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Bone Growth Stimulators Pipeline Market Report Overview
Bone growth stimulators are mainly used to facilitate bone growth in impaired or delayed healing fractures or fusions. They apply electrical or ultrasound waves to the site of the fracture or fusion.
The bone growth stimulators pipeline market research report provides comprehensive information about the bone growth stimulators pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
| Key Segments | · Implantable Bone Growth Stimulators
· External Bone Growth Stimulators |
| Key Territories | · The US
· Europe · Canada · Australia · Brazil |
| Key Regulatory Paths | · 510(k)
· CE · PMA · MDL · ANVISA · TGA |
| Leading Companies | · Bionext Biotech Products Ltd.
· Center for Biomedical Research Network in Bioengineering, Biomaterials and Nanomedicine · Curative Biosciences, Inc. · DJO Global Inc · Eepta Pty Ltd · GreenBone Ortho srl · Inion Oy · Johns Hopkins University · KeraCure, Inc. (Inactive) · Lattice Biologics Ltd |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Bone Growth Stimulators Pipeline Market by Segments
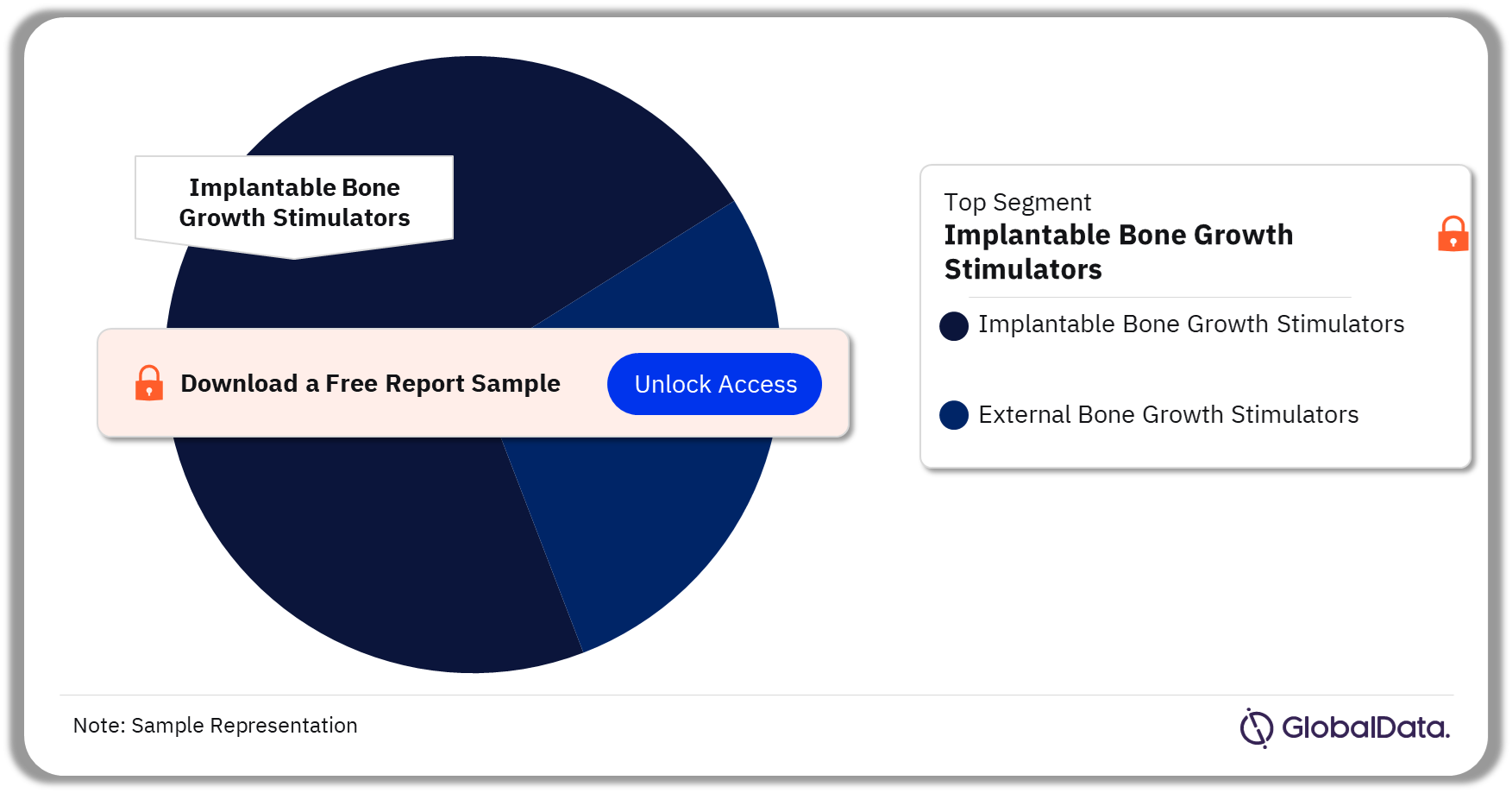
The key segments in the bone growth stimulators market are implantable bone growth stimulators and external bone growth stimulators. As of July 2023, implantable bone growth stimulator has a higher number of pipeline products. They are the stimulation devices that are implanted during surgery to stabilize the fracture. External bone growth stimulators are worn outside the skin and do not require any surgical procedure.
Bone Growth Stimulators Pipeline Market Analysis by Segments, 2023 (%)
For more segment insights into the bone growth stimulators pipeline market, download a free report sample
Bone Growth Stimulators Pipeline Market Segmentation by Territories
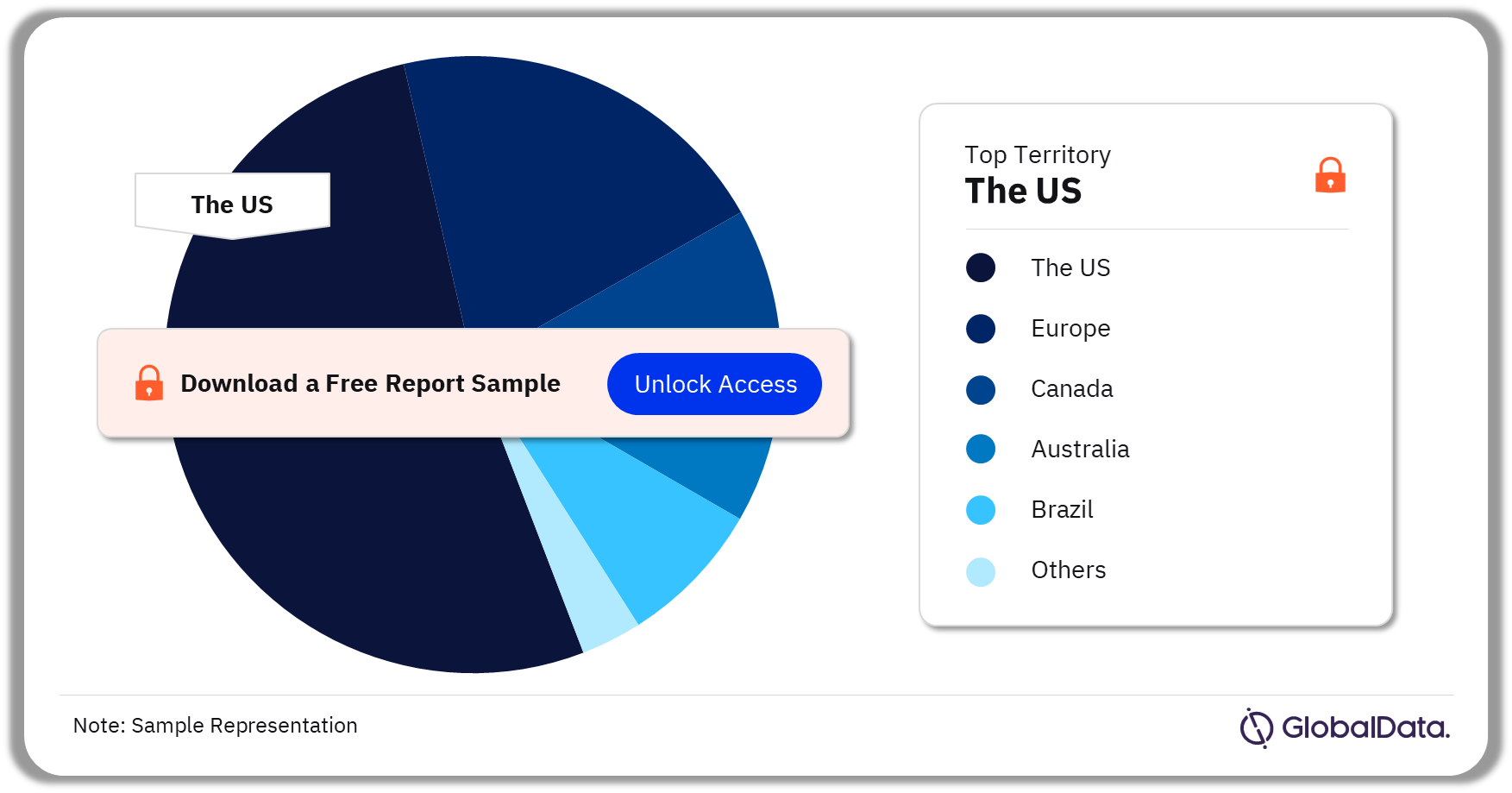
Some of the key territories in the bone growth stimulators market are the US, Europe, Canada, Australia, and Brazil. As of July 2023, the US has the highest number of pipeline products.
Bone Growth Stimulators Pipeline Market Analysis by Territories, 2023 (%)
For more territory insights into the bone growth stimulators pipeline market, download a free report sample
Bone Growth Stimulators Pipeline Market Segmentation by Regulatory Paths
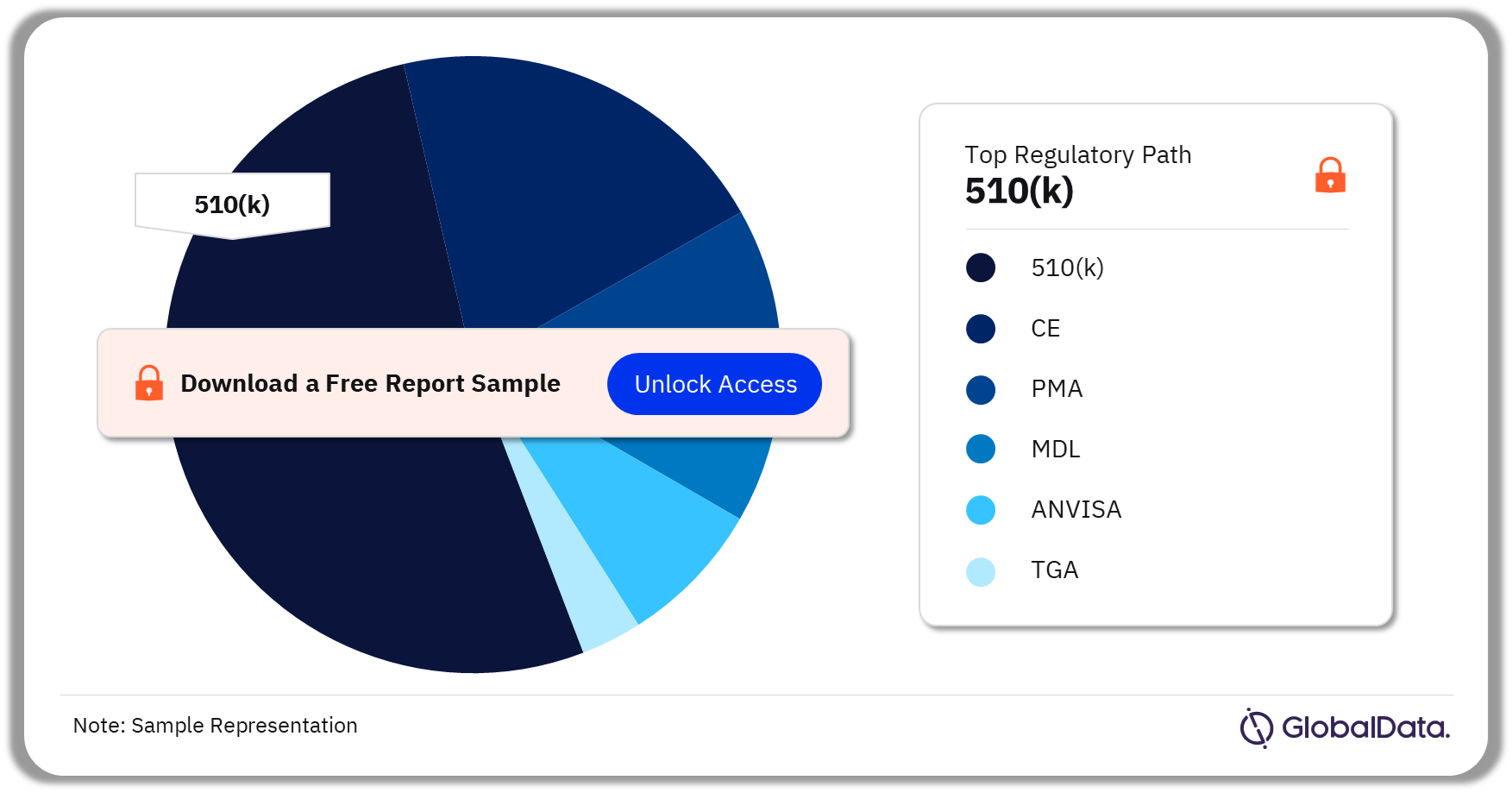
The key regulatory paths in the bone growth stimulators pipeline market are 510(k), CE, PMA, MDL, ANVISA, and TGA. As of July 2023, 510(k) is the most followed pathway for pipeline products in the market.
Bone Growth Stimulators Pipeline Market Analysis by Regulatory Paths, 2023 (%)
For more regulatory path insights into the bone growth stimulators pipeline market, download a free report sample
Bone Growth Stimulators Pipeline Market – Competitive Landscape
Some of the key companies in the bone growth stimulators pipeline market are DJO Global Inc, Inion Oy, Bionext Biotech Products Ltd., Center for Biomedical Research Network in Bioengineering, Biomaterials and Nanomedicine, Curative Biosciences, Inc., Eepta Pty Ltd, GreenBone Ortho srl, Johns Hopkins University, KeraCure, Inc. (Inactive), and Lattice Biologics Ltd.
DJO Global Inc: It is a medical device company that manufactures and distributes medical devices for musculoskeletal health, joint reconstruction, vascular health, and pain management. The company’s products include rigid and soft orthopedic bracing, bone growth stimulators, and hot and cold therapy, among others. DJO is headquartered in Lewisville, Texas, the US.
Inion Oy: It is a medical device company that develops biodegradable and bioactive implants. The company offers products that are used in specialty orthopedics, CMF surgeries, spine surgeries, arthroscopybone grafting, and dental surgery applications, among others. Inion is headquartered in Tampere, Finland.
Leading Bone Growth Stimulators Companies, 2023
For more company insights into the bone growth stimulators pipeline market, download a free report sample
Segments Covered in the Report
Bone Growth Stimulators Pipeline Market Segments Outlook
- Implantable Bone Growth Stimulators
- External Bone Growth Stimulators
Bone Growth Stimulators Pipeline Market Territories Outlook
- The US
- Europe
- Canada
- Australia
- Brazil
Bone Growth Stimulators Pipeline Market Regulatory Paths Outlook
- 510(k)
- CE
- PMA
- MDL
- ANVISA
- TGA
Scope
This report provides:
- Extensive coverage of the Bone Growth Stimulators under development.
- Details of major pipeline products which includes product description, licensing and collaboration details, and other developmental activities.
- Reviews of the major players involved in the development of Bone Growth Stimulators and lists of all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from early development to approved/issued stage.
- Key clinical trial data of ongoing trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies
- Identify emerging players with potentially strong product portfolios and create effective counter strategies to gain a competitive advantage
- Identify and understand important and diverse types of Bone Growth Stimulators under development
- Develop market-entry and market-expansion strategies
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date
Center for Biomedical Research Network in Bioengineering, Biomaterials and Nanomedicine
Curative Biosciences, Inc.
DJO Global Inc
Eepta Pty Ltd
GreenBone Ortho srl
Inion Oy
Johns Hopkins University
KeraCure, Inc. (Inactive)
Lattice Biologics Ltd
Magle Chemoswed AB
Max Biopharma Inc
NC Biomatrix BV
NDSU Research Foundation
Oklahoma State University
Oligo Medic Inc
Orthocell Ltd
Orthofix Medical Inc
OsteoGenix Incorporated (Inactive)
REGENECURE Ltd
Texas A&M University
The University of Nottingham
Universiti Putra Malaysia
University of California Davis
University of Florida
University of Wisconsin Madison
Wright Medical Group NV (Inactive)
ZetaGen Therapeutics Inc
ZimVie Inc
Table of Contents
Table
Figures
Frequently asked questions
-
Which is the leading segment in the bone growth stimulators pipeline market?
Implantable Bone Growth Stimulator is the leading segment in the bone growth stimulators pipeline market.
-
Which territory has the highest number of pipeline products in the bone growth stimulators pipeline market?
As of July 2023, the US has the highest number of pipeline products in the bone growth stimulators pipeline market.
-
Which is the most followed regulatory pathway in the bone growth stimulators pipeline market?
As of July 2023, 510(k) is the most followed pathway for pipeline products in the bone growth stimulators pipeline market.
-
Who are the major companies operating in the bone growth stimulators pipeline market?
Some of the key companies in the bone growth stimulators pipeline market are DJO Global Inc, Inion Oy, Bionext Biotech Products Ltd., Center for Biomedical Research Network in Bioengineering, Biomaterials and Nanomedicine, Curative Biosciences, Inc., Eepta Pty Ltd, GreenBone Ortho srl, Johns Hopkins University, KeraCure, Inc. (Inactive), and Lattice Biologics Ltd.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Bone Growth Stimulators reports