Bone Marrow Transplant Rejection Drugs in Development by Stages, Target, MoA, RoA, Molecule Type and Key Players, 2022 Update
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Bone Marrow Transplant Rejection Pipeline Drugs Market Report Overview
Bone Marrow Transplant Rejection is a complication that can occur after a stem cell or bone marrow transplant. The newly transplanted donor cells attack the transplant recipient’s body. Symptoms include chest pain, chills, drop in blood pressure, fever, flushing, funny taste in the mouth, headache, hives, nausea, pain and shortness of breath.
The Bone Marrow Transplant Rejection – drugs in development research report provides a comprehensive overview on the therapeutics under development for Bone Marrow Transplant Rejection, complete with analysis by stage of development, drug target, mechanism of action (MoA), route of administration (RoA) and molecule type. The report also covers the descriptive pharmacological action of the therapeutics, its complete research and development history and latest news and press releases. Additionally, the report provides an overview of key players involved in therapeutic development for Bone Marrow Transplant Rejection and features dormant and discontinued projects.
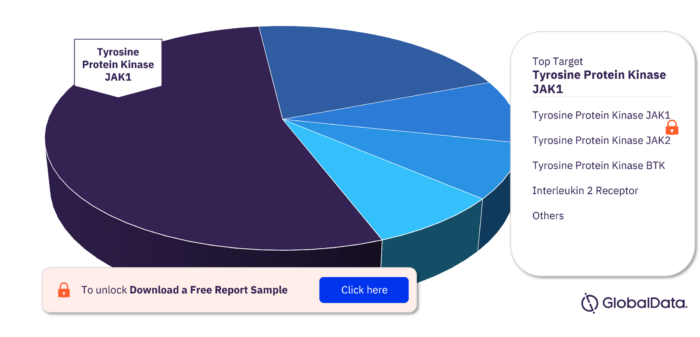
Key Targets in the Bone Marrow Transplant Rejection Pipeline Drugs Market
The key targets in the Bone Marrow Transplant Rejection pipeline drugs market are Tyrosine Protein Kinase JAK1, Tyrosine Protein Kinase JAK2, Tyrosine Protein Kinase BTK, Interleukin 2 Receptor, Programmed Cell Death Protein 1, Alpha 1 Antitrypsin, Cells Expressing B Lymphocyte Antigen CD20, Interleukin 2 Receptor Subunit Beta, Macrophage Colony Stimulating Factor 1 Receptor, and Tyrosine Protein Kinase ITK/TSK among others.
Bone Marrow Transplant Rejection Pipeline Drugs Market, by Targets
For more target insights, download a free report sample
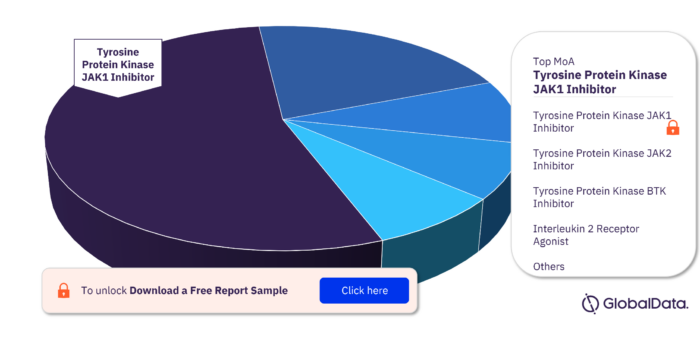
Key MoA in the Bone Marrow Transplant Rejection Pipeline Drugs Market
The key mechanisms of action in the Bone Marrow Transplant Rejection pipeline drugs market are Tyrosine Protein Kinase JAK1 Inhibitor, Tyrosine Protein Kinase JAK2 Inhibitor, Tyrosine Protein Kinase BTK Inhibitor, Interleukin 2 Receptor Agonist, Alpha 1 Antitrypsin Replacement, Cytotoxic To Cells Expressing B Lymphocyte Antigen CD20 , Interleukin 2 Receptor Subunit Beta Antagonist, Programmed Cell Death Protein 1 Agonist, Tyrosine Protein Kinase ITK/TSK Inhibitor, and CD40 Ligand Inhibitor among others.
Bone Marrow Transplant Rejection Pipeline Drugs Market, by MoA
To get more insights on key MoA, download a free sample report
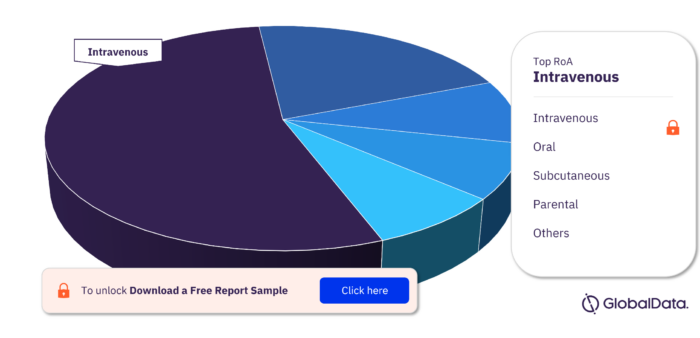
Bone Marrow Transplant Rejection Pipeline Drugs Market Segmentation by RoA
The key routes of administration in the Bone Marrow Transplant Rejection pipeline drugs market are Intravenous, Oral, Subcutaneous, Parenteral, Topical, Intracoronary, Intramuscular, Hemodialysis, Intraarticular, Intralesional, and Intraperitoneal.
Bone Marrow Transplant Rejection Pipeline Drugs Market Analysis, by RoA
To get more insights on key RoA, download a free sample report
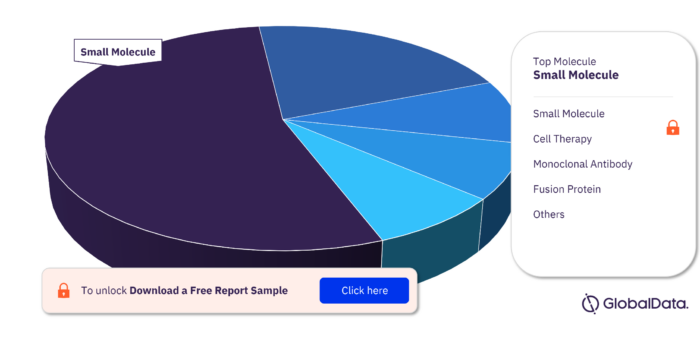
Key Molecule Types in the Bone Marrow Transplant Rejection Pipeline Drugs Market
The key molecule types in the Bone Marrow Transplant Rejection pipeline drugs market are Small Molecule, Cell Therapy, Monoclonal Antibody, Fusion Protein, Recombinant Protein, Biologic, Gene-Modified Cell Therapy, Antibody, Blood Derivative, and Synthetic Peptide among others.
Bone Marrow Transplant Rejection Pipeline Drugs Market, by Molecule Type
To get more insights on key molecule types, download a free sample report
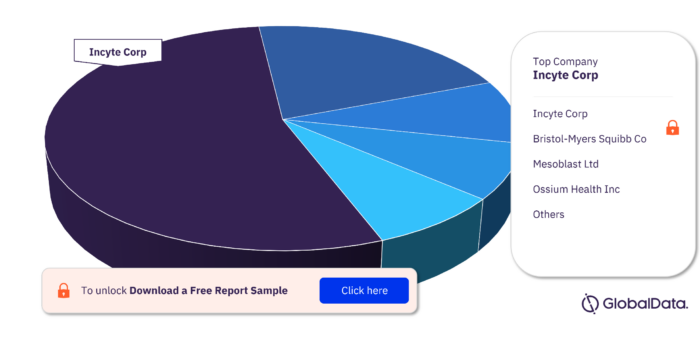
Major Companies in the Bone Marrow Transplant Rejection Pipeline Drugs Market
The major companies in the Bone Marrow Transplant Rejection pipeline drugs market are Incyte Corp, Bristol-Myers Squibb Co, Mesoblast Ltd, Ossium Health Inc, Pfizer Inc, AbbVie Inc, AnaptysBio Inc, AstraZeneca Plc, Avalon GloboCare Corp, Biocon Ltd, CheckPoint Immunology Inc, Cynata Therapeutics Ltd, and EV Therapeutics Inc among others.
Bone Marrow Transplant Rejection Pipeline Drugs Market, by Major Companies
For more company insights, download a free sample report
Bone Marrow Transplant Rejection Pipeline Drugs Market Overview
| Key Targets | Tyrosine Protein Kinase JAK1, Tyrosine Protein Kinase JAK2, Tyrosine Protein Kinase BTK, Interleukin 2 Receptor, Programmed Cell Death Protein 1, Alpha 1 Antitrypsin, Cells Expressing B Lymphocyte Antigen CD20, Interleukin 2 Receptor Subunit Beta, Macrophage Colony Stimulating Factor 1 Receptor, and Tyrosine Protein Kinase ITK/TSK |
| Key Mechanisms of action | Tyrosine Protein Kinase JAK1 Inhibitor, Tyrosine Protein Kinase JAK2 Inhibitor, Tyrosine Protein Kinase BTK Inhibitor, Interleukin 2 Receptor Agonist, Alpha 1 Antitrypsin Replacement, Cytotoxic To Cells Expressing B Lymphocyte Antigen CD20 , Interleukin 2 Receptor Subunit Beta Antagonist, Programmed Cell Death Protein 1 Agonist, Tyrosine Protein Kinase ITK/TSK Inhibitor, and CD40 Ligand Inhibitor |
| Key Routes of Administration | Intravenous, Oral, Subcutaneous, Parenteral, Topical, Intracoronary, Intramuscular, Hemodialysis, Intraarticular, Intralesional, and Intraperitoneal |
| Key Molecule Types | Small Molecule, Cell Therapy, Monoclonal Antibody, Fusion Protein, Recombinant Protein, Biologic, Gene-Modified Cell Therapy, Antibody, Blood Derivative, and Synthetic Peptide |
| Major Companies | Incyte Corp, Bristol-Myers Squibb Co, Mesoblast Ltd, Ossium Health Inc, Pfizer Inc, AbbVie Inc, AnaptysBio Inc, AstraZeneca Plc, Avalon GloboCare Corp, Biocon Ltd, CheckPoint Immunology Inc, Cynata Therapeutics Ltd, and EV Therapeutics Inc |
Scope
- The pipeline guide provides a snapshot of the global therapeutic landscape of Bone Marrow Transplant Rejection
- The pipeline guide reviews pipeline therapeutics for Bone Marrow Transplant Rejection by companies and universities/research institutes based on information derived from company and industry-specific sources.
- The pipeline guide covers pipeline products based on several stages of development ranging from pre-registration till discovery and undisclosed stages.
- The pipeline guide features descriptive drug profiles for the pipeline products which comprise, product description, descriptive licensing and collaboration details, R&D brief, MoA & other developmental activities.
- The pipeline guide reviews key companies involved in Bone Marrow Transplant Rejection therapeutics and enlists all their major and minor projects.
- The pipeline guide evaluates Bone Marrow Transplant Rejection therapeutics based on mechanism of action (MoA), drug target, route of administration (RoA), and molecule type.
- The pipeline guide encapsulates all the dormant and discontinued pipeline projects.
- The pipeline guide reviews the latest news related to pipeline therapeutics for Bone Marrow Transplant Rejection
Reasons to Buy
- Procure strategically important competitor information, analysis, and insights to formulate effective R&D strategies.
- Recognize emerging players with a potentially strong product portfolio and create effective counter-strategies to gain a competitive advantage.
- Find and recognize significant and varied types of therapeutics under development for Bone Marrow Transplant Rejection
- Classify potential new clients or partners in the target demographic.
- Develop tactical initiatives by understanding the focus areas of leading companies.
- Plan mergers and acquisitions meritoriously by identifying key players and their most promising pipeline therapeutics.
- Formulate corrective measures for pipeline projects by understanding Bone Marrow Transplant Rejection pipeline depth and focus of Indication therapeutics.
- Develop and design in-licensing and out-licensing strategies by identifying prospective partners with the most attractive projects to enhance and expand business potential and scope.
- Adjust the therapeutic portfolio by recognizing discontinued projects and understand from the know-how what drove them from the pipeline.
Abbisko Cayman Limited
AbbVie Inc
Accro BioScience (Suzhou) Co Ltd
Actitrexx GmbH
Adienne Pharma & Biotech SA
Alphamab Oncology
AltruBio Inc
Amcyte Pharma Inc
Amgen Inc
AnaMar AB
AnaptysBio Inc
ASC Therapeutics Inc
AstraZeneca Plc
Athos Therapeutics Inc
Autolus Therapeutics Plc
Avalon GloboCare Corp
Bellicum Pharmaceuticals Inc
Biocon Ltd
Biogen Inc
BioIncept LLC
BlueRock Therapeutics LLC
BlueSphere Bio Inc
Boryung ViGenCell Inc
Bristol-Myers Squibb Co
Capricor Therapeutics Inc
Cell Source Inc
Cellective BioTherapy Inc
Cellenkos Inc
Cellestia Biotech AG
Cellix Bio Pvt Ltd
CELLnLIFE Inc
Celltrion Inc
Cellvation Inc
CheckPoint Immunology Inc
Claritas Pharmaceuticals Inc
Clinigen Group Plc
Compugen Ltd
Connext Co Ltd
Corvus Pharmaceuticals Inc
CSL Ltd
CTI BioPharma Corp
Cue Biopharma Inc
Cynata Therapeutics Ltd
Cytopeutics Pte Ltd
Daewoong Pharmaceutical Co Ltd
Dianomi Therapeutics Inc
Dualogics Corp
Educell doo
Eli Lilly and Co
enGene Inc
Equillium Inc
EV Therapeutics Inc
Evive Biotech
ExCellThera Inc
F. Hoffmann-La Roche Ltd
Faron Pharmaceuticals Oy
Fate Therapeutics Inc
Genentech USA Inc
Gilead Sciences Inc
GlaxoSmithKline Plc
Glia LLC
Good T Cells Inc
Grifols SA
Hangzhou East China Pharmaceutical Group Co Ltd
Humanigen Inc
iCELL Biotechnology Co Ltd
Immplacate Inc
Immune Modulation Inc
ImmuneTarget Inc
Imstem Biotechnology Inc
Incyte Corp
Inmagene Biopharmaceuticals Ltd
ITB-Med AB
Jazz Pharmaceuticals Plc
JN Biosciences LLC
Kadmon Holdings Inc
Kamada Pharmaceuticals
Kiniksa Pharmaceuticals Ltd
Kymab Ltd
MaaT Pharma
Machavert Pharmaceuticals LLC
Magenta Therapeutics Inc
Mallinckrodt Plc
Medexus Pharmaceuticals Inc
Medicenna Therapeutics Corp
Mediolanum farmaceutici SpA
Medsenic SAS
Merck & Co Inc
Mereo Biopharma Group Plc
Mesoblast Ltd
Millennium Pharmaceuticals Inc
MiNK Therapeutics Inc
MitoImmune Therapeutics Inc
NapaJen Pharma Inc
Neoleukin Therapeutics Inc
Neovii Pharmaceuticals AG
Nurix Therapeutics Inc
OncoC4 Inc
Orca Biosystems Inc
OSE Immunotherapeutics
Ossium Health Inc
Panorama Researchama Research
Pfizer Inc
Platinum Biotech (Beijing) Co Ltd
Pluristem Therapeutics Inc
Precision Biosciences Inc
Rebus Holdings Inc
REGiMMUNE Corp
Rheos Medicines Inc
SCM lifescience Co Ltd
Seagen Inc
Secura Bio Inc
Seres Therapeutics Inc
Shenzhen Chipscreen Biosciences Co Ltd
Sino Biopharmaceutical Ltd
Sorrento Therapeutics Inc
STERO Biotechs Ltd
StingInn LLC
Suzhou Connect Biopharmaceuticals Ltd
Suzhou Regend Therapeutics Co Ltd
Suzhou Zelgen Biopharmaceutical Co Ltd
Synthetic Biologics Inc
Takeda Pharmaceutical Co Ltd
Targazyme Inc
TCF GmbH
TCR2 Therapeutics Inc
TeraImmune Inc
Tianjin Angsai Cell Genetic Engineering Co Ltd
Trailhead Biosystems Inc
TreeFrog Therapeutics SAS
Triursus Therapeutics Inc
United BioPharma Inc
Vault Pharma Inc
VectivBio Holding AG
Vedanta Biosciences Inc
Viracta Therapeutics Inc
Visterra Inc
VITRAC Therapeutics LLC
Xbiome Co Ltd
Xenikos BV
Xenothera SAS
Table of Contents
Table
Figures
Frequently asked questions
-
What are the key targets in the Bone Marrow Transplant Rejection pipeline drugs market?
The key targets in the Bone Marrow Transplant Rejection pipeline drugs market are Tyrosine Protein Kinase JAK1, Tyrosine Protein Kinase JAK2, Tyrosine Protein Kinase BTK, Interleukin 2 Receptor, Programmed Cell Death Protein 1, Alpha 1 Antitrypsin, Cells Expressing B Lymphocyte Antigen CD20, Interleukin 2 Receptor Subunit Beta, Macrophage Colony Stimulating Factor 1 Receptor, and Tyrosine Protein Kinase ITK/TSK.
-
What are the key mechanisms of action in the Bone Marrow Transplant Rejection pipeline drugs market?
The key mechanisms of action in the Bone Marrow Transplant Rejection pipeline drugs market are Tyrosine Protein Kinase JAK1 Inhibitor, Tyrosine Protein Kinase JAK2 Inhibitor, Tyrosine Protein Kinase BTK Inhibitor, Interleukin 2 Receptor Agonist, Alpha 1 Antitrypsin Replacement, Cytotoxic To Cells Expressing B Lymphocyte Antigen CD20 , Interleukin 2 Receptor Subunit Beta Antagonist, Programmed Cell Death Protein 1 Agonist, Tyrosine Protein Kinase ITK/TSK Inhibitor, and CD40 Ligand Inhibitor.
-
What are the key routes of administration in the Bone Marrow Transplant Rejection pipeline drugs market?
The key routes of administration in the Bone Marrow Transplant Rejection pipeline drugs market are Intravenous, Oral, Subcutaneous, Parenteral, Topical, Intracoronary, Intramuscular, Hemodialysis, Intraarticular, Intralesional, and Intraperitoneal.
-
What are the key molecule types in the Bone Marrow Transplant Rejection pipeline drugs market?
The key molecule types in the Bone Marrow Transplant Rejection pipeline drugs market are Small Molecule, Cell Therapy, Monoclonal Antibody, Fusion Protein, Recombinant Protein, Biologic, Gene-Modified Cell Therapy, Antibody, Blood Derivative, and Synthetic Peptide.
-
What are the major companies in the Bone Marrow Transplant Rejection pipeline drugs market?
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Immunology reports